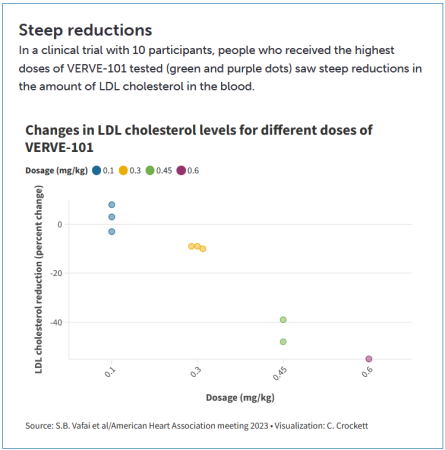
Analyze a clinical trial’s result
This exercise is a part of Educator Guide: Analyze a Clinical Trial and Horned Reptiles / View Guide
Direction for teachers:
This lesson plan includes two sets of graphical analysis questions followed by a...