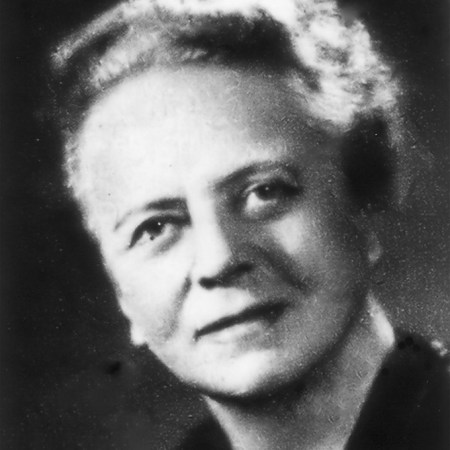Connie Zhou/OTTO
Peering inside the atom
Matter is a lush tapestry, woven from a complex assortment of threads. Diverse varieties of subatomic particles intertwine to fabricate the universe we inhabit. But a century ago, people believed that matter was so simple that it could be constructed with just two types of subatomic fibers — electrons and protons. That vision of matter was a no-nonsense plaid instead of an ornate brocade.
Physicists of the 1920s thought they had a solid grasp on what made up matter. They knew that atoms contained electrons surrounding a positively charged nucleus. And they knew that each nucleus contained a number of protons, positively charged particles identified in 1919. Combinations of those two particles made up all of the matter in the universe, it was thought. That went for everything that ever was or might be, across the vast, unexplored cosmos and at home on Earth.
The scheme was appealingly tidy, but it swept under the carpet a variety of hints that all was not well in physics. Two discoveries in one revolutionary year, 1932, would force physicists to peek underneath the rug. First, the discovery of the neutron unlocked new ways to peer into the hearts of atoms and even split them in two. Then came news of the positron — identical to the electron but with the opposite charge. Its discovery would foreshadow many more surprises to come. Additional particle discoveries ushered in a new framework for the fundamental bits of matter, now known as the standard model.
And that “annus mirabilis” — miraculous year — would set physicists on two parallel tracks of exploration. One would blossom into the modern discipline of particle physics. After the positron’s appearance, the discovery of dozens more particles would lead to a new insight: Protons and neutrons aren’t elementary. They have even smaller components called quarks. Particle physics scrutinizes those most fundamental bits of matter — quarks, electrons, positrons and the like.
The other track would lead to modern nuclear physics, concerned with the workings of atoms’ hearts, how they decay, transform and react. Discoveries there would put scientists on a trajectory toward a most devastating technology: nuclear weapons. The bomb would cement the importance of science — and science journalism — in the public eye, says nuclear historian Alex Wellerstein of Stevens Institute of Technology in Hoboken, N.J. “The atomic bomb becomes the ultimate proof that … indeed this is world-changing stuff.”
In the decades that followed, these fields would fundamentally alter how humankind understood and manipulated matter. Soon, physicists were busier than ever.
— Emily Conover
Goodbye, two particles
Physicists of the 1920s embraced a particular type of conservatism. Embedded deep in their psyches was a reluctance to declare the existence of new particles. Researchers stuck to the status quo of matter composed solely of electrons and protons. It’s an idea that has been dubbed the “two-particle paradigm,” and it held until about 1930. In that time period, says historian of science Helge Kragh of the University of Copenhagen, “I’m quite sure that not a single mainstream physicist came up with the idea that there might exist more than two particles.” The utter simplicity of two particles explaining everything nature’s bounty could produce was so appealing to physicists’ sensibilities that they found the idea difficult to let go of.
The paradigm held back theoretical descriptions of the neutron and the positron, two particles found one after the other in 1932. And another would-be new particle, the neutrino, proposed in 1930, was likewise considered unappealing. “To propose the existence of other particles was widely regarded as reckless and contrary to the spirit of Occam’s razor,” science biographer Graham Farmelo wrote in Contemporary Physics in 2010.
Still, during the early 20th century, physicists were diligently investigating a few puzzles of matter that would, after some hesitation, inevitably lead to new particles. These included unanswered questions about the details of radioactive decay, the identities and origins of energetic particles called cosmic rays, and why chemical elements occur in different varieties called isotopes, which have similar chemical properties but varying masses.
New Zealand–born British physicist Ernest Rutherford stopped just short of positing a fundamentally new particle in 1920. He realized that neutral particles in the nucleus could explain the existence of isotopes. Such particles came to be known as “neutrons.” But rather than proposing that neutrons were fundamentally new, he thought they were composed of protons combined in close proximity with electrons to make neutral particles. He was correct about the role of the neutron, but wrong about its identity.
Rutherford’s idea was convincing, British physicist James Chadwick recounted in a 1969 interview, “The only question was how the devil could one get evidence for it.” The neutron’s lack of electric charge made it a particularly wily target. In between work on other projects, Chadwick began hunting for the particles at Cavendish Laboratory at the University of Cambridge, then led by Rutherford. Chadwick found his evidence in 1932 — reporting that mysterious radiation emitted when beryllium was bombarded with the nuclei of helium atoms could be explained by a particle with no charge and with a mass similar to the proton’s. In other words, a neutron. Chadwick didn’t expect the important role his discovery would play. “I am afraid neutrons will not be of any use to anyone,” he told the New York Times shortly after his discovery.
Physicists would grapple with the neutron’s identity over the following years before accepting it as an entirely new particle, not the amalgamation that Rutherford had suggested. For one, a proton-electron mash-up conflicted with the young theory of quantum mechanics, which characterizes physics on small scales. The Heisenberg uncertainty principle — which states that if the location of an object is well known, its momentum cannot be — suggests an electron confined within a nucleus would have an unreasonably large energy. And certain nuclei’s spins, a quantum mechanical measure of angular momentum, likewise suggested that the neutron was a full-fledged particle, as did improved measurements of the particle’s mass.
Physicists also resisted the positron, until it became difficult to ignore.
The positron’s 1932 detection had been foreshadowed by the work of British theoretical physicist Paul Dirac. But it took some floundering about before physicists realized the meaning of his work. In 1928 Dirac formulated an equation that combined quantum mechanics and the special theory of relativity, formulated by Albert Einstein back in 1905, which describes physics close to the speed of light. Now known simply as the Dirac equation, the expression explained the behavior of electrons in a way that satisfied both theories.
But the equation suggested something odd: the existence of another type of particle, one with the opposite electric charge. At first, Dirac and other physicists clung to the idea that this charged particle might be the proton. But the two particles should have the same mass, and protons are almost 2,000 times as heavy as electrons. In 1931 Dirac proposed a new particle, with the same mass as the electron but with opposite charge.
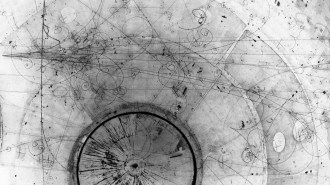
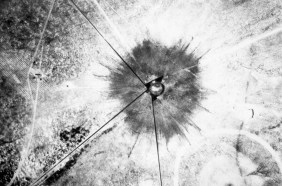
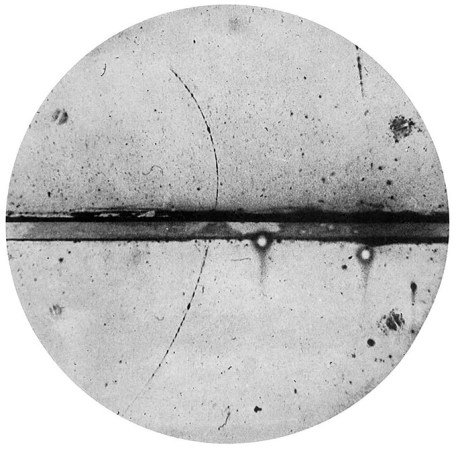
Meanwhile, American physicist Carl Anderson of Caltech, independent of Dirac’s work, was using a device called a cloud chamber to study energetic particles originating in space, called cosmic rays. Cosmic rays, discovered in 1912, fascinated scientists because they didn’t fully understand what the particles were or how they were produced. Within Anderson’s chamber, liquid droplets condensed along the paths of energetic charged particles, a result of the particles ionizing gas molecules as they zipped along. The experiments revealed positively charged particles with masses equal to an electron’s. Soon, the connection to Dirac’s theory became clear.
Science News Letter, the predecessor of Science News, had a hand in naming the newfound particle. Editor Watson Davis proposed “positron” in a telegram to Anderson, who had independently considered the moniker, according to a 1933 Science News Letter article. In a 1966 interview, Anderson recounted mulling over Davis’ suggestion during a game of bridge, and finally going along with it. But he later regretted the choice, saying in the interview, “I think that’s a very poor name.”

The discovery of the positron, the antimatter partner of the electron, marked the advent of antimatter research. And today, antimatter’s existence still seems baffling. Every object we can see and touch is made of matter, making antimatter seem downright extraneous. Antimatter’s lack of relevance to daily life — and the liberal use of the term in Star Trek — means that many nonscientists still envision it as the stuff of science fiction. But even a banana sitting on a counter emits antimatter multiple times a day, periodically spitting out positrons in radioactive decays of the potassium it contains.
Physicists would go on to discover many other antiparticles — all of which are identical to their matter partners except for an opposite electric charge — including the antiproton in 1955. The subject still keeps physicists up at night. Scientists think the Big Bang should have produced equal amounts matter and antimatter, so researchers today are studying how antimatter became rare.
In the 1930s, antimatter was such a leap that Dirac’s hesitation to propose the positron was understandable. Not only would the positron break the two-particle paradigm, but it would also suggest that electrons had mirror images with no apparent role in making up atoms. When asked, decades later, why he had not predicted the positron after he first formulated his equation, Dirac replied, “pure cowardice.”
The discoveries came on the heels of yet another particle prediction, the neutrino. Reluctantly postulated by Austrian physicist Wolfgang Pauli in 1930, the particle has no electric charge and interacts very rarely, suggesting it would never be detected: “I have done something very bad today by proposing a particle that cannot be detected; it is something no theorist should ever do,” Pauli reportedly said.
Pauli’s prediction was what he called a “desperate remedy.” Researchers studying a type of radioactive decay known as beta decay had hit on a quandary that threatened to undermine the basics of physics. In beta decay, an atom spits out an electron and converts into a different element. A central physics principle, conservation of energy, suggests that the particles emitted in radioactive decays from identical atoms should always carry the same amount of energy. But the wayward electrons had a range of energies. That apparent noncompliance led some physicists to propose the radical idea that energy was not always conserved.
In a letter to a group of nuclear physicists, which Pauli famously addressed, “Dear radioactive ladies and gentlemen,” he proposed that the electron in beta decay was accompanied by a second, undetected particle that would carry away some energy.
Soon, Italian physicist Enrico Fermi would popularize the name “neutrino” for the particle, Italian for “little neutral one.” In 1934 he would come up with a mathematical theory based on the particle’s existence that successfully described beta decay. In Fermi’s scheme, the electron and neutrino were released when a neutron converted into a proton in the atom’s nucleus. That general interpretation still stands, though today’s physicists now refer to the particle as an electron antineutrino, because three types of neutrinos and their antiparticles are now known. Fermi’s explanation bolstered belief in neutrinos, conclusively detected in 1956.
So by the mid-1930s, the two-particle paradigm was out. Physicists’ understanding had advanced, but their austere vision of matter had to be jettisoned. That shift in mindset would soon be reinforced with even more particle discoveries, and the simple picture of nature was further demolished.
— Emily Conover

Support our next century
100 years after our founding, top-quality, fiercely accurate reporting on key advances in science, technology, and medicine has never been more important – or more in need of your support. The best way to help? Subscribe.
Many, many particles
Physicists have long revered elegance, expecting that nature at its most basic should be simple. That sensibility was evident in the 1920s insistence that the electron and proton made up all of matter. But after the conceptual logjam against new particles broke down, and technological advances opened up new ways to explore the subatomic realm, physicists found themselves drowning in a flood of new particles. Fleshing out an explanation would consume physicists for decades.
In the 1950s and ’60s, new particles were detected by the dozens, forming an alphabet soup of Greek letters: phi baryons, xi baryons, eta mesons and many, many more. “If I could remember the names of all these particles I’d be a botanist,” physicist Enrico Fermi famously said. Many of these newfound particles were exotic varieties, formed when particles collide at high energies and not present within atoms.
Physicists quickly tired of the deluge. In 1955, physicist Willis Lamb, Jr. recounted a saying of the time, “the finder of a new elementary particle used to be rewarded by a Nobel Prize, but such a discovery now ought to be punished by a $10,000 fine.”
At first, the particles were found gradually, by studying natural collisions, produced from energetic particles from space called cosmic rays. But in the mid-1950s, particle accelerators kicked things up a notch. With this new technology, physicists could boost beams of particles to high speeds, smashing them into targets or other beams of particles, to see what might emerge from the smashups.

At the time, scientists couldn’t explain why so many apparently fundamental particles existed, especially given that everyday matter required only protons, neutrons and electrons. “By 1960 or so it was a widespread feeling that there were too many particles, and that there had to be some family resemblance between them,” says historian of science Helge Kragh of the University of Copenhagen. Soon the concept of quarks would bring some order to the menagerie.
As Science News Letter reported in 1964, “A quark is not an animal out of Alice in Wonderland or the sound a duck might make.” Instead, quarks, proposed in 1964 and confirmed in experiments over the next decade, are smaller particles that, mashed together in different combinations, make up many of the particles previously considered fundamental, including protons and neutrons.
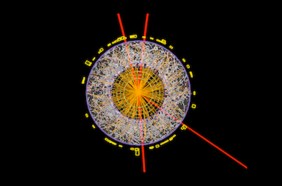
Additional work led physicists to a coherent picture of the fundamental particles and forces of nature, called the standard model. The work of many physicists operating independently and in groups, the framework consists of 17 particles, plus antiparticle partners. Included on the list are six types of quarks and six leptons. Electrons and their heavier relatives, muons and taus, are leptons, as are a trio of lightweight particles called neutrinos. Rounding out the crew are bosons, which, among others, include the particles of light called photons and the Higgs boson, which explains the origin of particles’ mass.
Spotlight
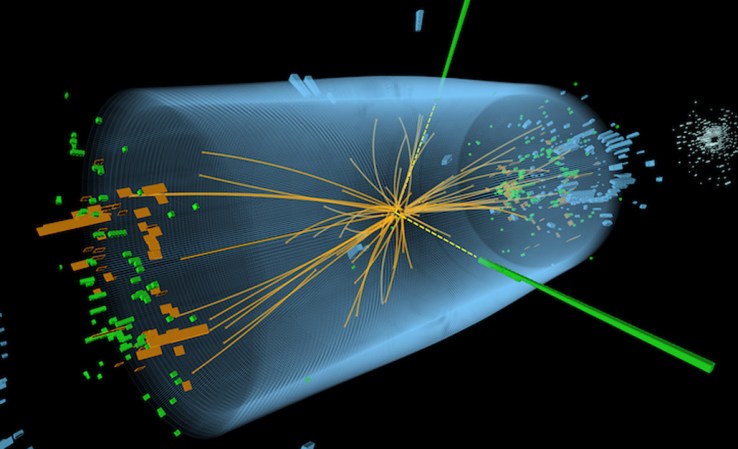
How particle detectors capture matter’s hidden, beautiful reality
Old and new detectors trace the whirling paths of subatomic particles.
All the standard particles
The standard model is the theory of the fundamental particles and forces of nature. It includes 12 particles that make up the material world: matter, shown on the left of this diagram, and their antimatter partners. Another four particles (right) transmit the forces of nature and one, the Higgs boson, results from the process by which fundamental particles gain mass.
Tap the colored sections below for more.
The standard model also accounts for three of the four known fundamental forces: electromagnetism, the weak nuclear force and the strong nuclear force. The weak force governs certain radioactive decays, and the strong force holds quarks together inside particles. (One of nature’s most familiar forces, gravity, is not yet incorporated into the framework.)
Four decades passed between the establishment of the standard model in the 1970s and the detection of all its particles. The effort to find each and every particle required a succession of increasingly larger and more energetic particle accelerators, to unleash more exotic particles and those with higher masses. Accelerators advanced from a few billions of electron volts in the mid-1950s to the trillions of electron volts needed to discover the final predicted standard model particle, the Higgs boson, found with the Large Hadron Collider at CERN near Geneva in 2012.
Physicists frequently describe the standard model as one of the most successful theories ever created, as it has correctly predicted a wide variety of experimental results. But despite the successes, physicists can’t explain why its various fundamental particles and forces exist.
“The standard model is a great thing … but it leaves unanswered an enormous number of questions, and … in so far as the theory can talk it says, ‘I can’t answer those questions. Find something better,’ ” physicist Sheldon Glashow said in a 1998 interview.
Physicists have been searching for that “something better” by studying potential additions or modifications to the standard model. For example, an idea called supersymmetry gained traction in the 1970s, proposing that every known particle has a heavier partner. Among other appealing characteristics, supersymmetry, if it exists, could reveal that the standard model’s three fundamental forces are actually different aspects of one unified force.
But so far, there’s no evidence for supersymmetry or any other modifications to the standard model. Adding to the frustration is the clear evidence that the standard model can’t explain everything. For example, cosmic observations suggest the universe contains an unidentified type of matter, known as dark matter. Though evidence of its existence came in 1933, just a year after the neutron’s discovery, dark matter remains an enduring puzzle, showing that scientists still have more to learn about the foundations of matter. After decades of searches with increasingly more sensitive detectors, a favored class of hypothetical dark matter particles called WIMPs have failed to turn up. Yet there’s still hope: A new generation of experiments is beginning, and searches for another proposed type of dark matter particles, called axions, are just getting going.
Particle physicists are now struggling to move forward. Some are pushing for even bigger colliders, but such projects may be prohibitively expensive. Dark matter hunters and others are focused on performing highly precise experiments looking for rare, subtle effects that might hint at a new theory. And some are studying the strange behavior of neutrinos, which could reveal new secrets about the differences between matter, common in the universe, and the rarer antimatter.
One of these tactics, physicists hope, will lead to a new, simpler, more satisfying theory of matter.
— Emily Conover
Spotlight
The dark matter mystery deepens with the demise of a reported detection
Early results from an experiment designed to replicate one that hinted that dark matter is made up of WIMPs came up empty-handed.
Unleashing the atom

Radioactive decay hints that atoms hold stores of energy locked within, ripe for the taking. Although radioactivity was discovered in 1896, that energy long remained an untapped resource. The neutron’s discovery in the 1930s would be key to unlocking that energy — for better and for worse.
Opening up a better understanding of the nucleus, the neutron’s discovery gave scientists new abilities to split atoms into two or transform them into other elements. Developing that nuclear know-how led to useful technologies, like nuclear power, but also devastating nuclear weapons.
Just a year after the neutron was found, Hungarian-born physicist Leo Szilard envisioned using neutrons to split atoms and create a bomb. “[I]t suddenly occurred to me that if we could find an element which is split by neutrons and which would emit two neutrons when it absorbed one neutron, such an element, if assembled in sufficiently large mass, could sustain a nuclear chain reaction, liberate energy on an industrial scale, and construct atomic bombs,” he later recalled. It was a fledgling idea, but prescient.
Because neutrons lack electric charge, they can penetrate atoms’ hearts. In 1934, physicist Enrico Fermi and colleagues started bombarding dozens of different elements with neutrons, producing a variety of new, radioactive isotopes. Each isotope of a particular element contains a different number of neutrons in its nucleus, with the result that some isotopes may be radioactive while others are stable. Fermi had been inspired by another striking discovery of the time. In 1934 French chemists Frédéric and Irène Joliot-Curie reported the first artificially created radioactive isotopes, produced by bombarding elements with helium nuclei, called alpha particles. Now, Fermi was doing something similar, but with a more penetrating probe.
There were a few scientific missteps on the way to understanding the results of such experiments. A major goal was to produce brand-new elements, those beyond the last known element in the periodic table at that time, uranium. After blasting uranium with neutrons, Fermi and colleagues reported evidence of success. But that conclusion would turn out to be incorrect.
German chemist Ida Noddack had an inkling that all was not right with Fermi’s interpretation. She came close to the correct explanation for his experiments in a 1934 paper, writing, “When heavy nuclei are bombarded by neutrons, it is conceivable that the nucleus breaks up into several large fragments.” But Noddack didn’t follow up on the idea. “She didn’t provide any kind of supporting calculation and nobody took it with much seriousness,” says physicist Bruce Cameron Reed of Alma College in Michigan.
In Germany, physicist Lise Meitner and chemist Otto Hahn had also begun bombarding uranium with neutrons. But Meitner, an Austrian of Jewish heritage in increasingly hostile Nazi Germany, was forced to flee in July 1938. She had only an hour and a half to pack her suitcases. Hahn and a third member of the team, chemist Fritz Strassmann, continued the work, corresponding from afar with Meitner, who had landed in Sweden. The results of the experiments were puzzling at first, but when Hahn and Strassmann reported to Meitner that barium — a much lighter element than uranium — was a product of the reaction, it became clear what was happening. The nucleus was splitting.
Meitner and her nephew, physicist Otto Frisch, worked together to explain the phenomenon, a process the pair would call “fission.” Hahn received the 1944 Nobel Prize in chemistry for the discovery of fission, but Meitner never won a Nobel, in a decision now widely considered unjust. Meitner was nominated for the prize in either physics or chemistry a whopping 48 times, most after the discovery of fission. “Her peers in the physics community recognized that she was part of the discovery,” says chemist Ruth Lewin Sime of Sacramento City College, who has written extensively about Meitner. “That included just about anyone who was anyone.”

Word of the discovery soon spread, and on January 26, 1939, renowned Danish physicist Niels Bohr publicly announced at a scientific meeting that fission had been achieved. The potential implications were immediately apparent: Fission could unleash the energy stored in atomic nuclei, potentially resulting in a bomb. A Science News Letter story describing the announcement attempted to dispel any concerns the discovery might raise. The article, titled “Atomic energy released,” reported that scientists “are fearful lest the public become worried about a ‘revolution’ in civilization as a result of their researches,” such as “the suggested possibility that the atomic energy may be used as some super-explosive, or as a military weapon.” But downplaying the catastrophic implications didn’t prevent them from coming to pass.
The question of whether a bomb could be created rested, once again, on neutrons. For fission to ignite an explosion, it would be necessary to set off a chain reaction. That means each fission would release additional neutrons, which could then go on to induce more fissions, and so on. Experiments quickly revealed that enough neutrons were released to make such a chain reaction feasible.
Spotlight
How understanding nature made the atomic bomb inevitable
On the anniversary of Hiroshima, here’s a look back at the chain reaction of basic discoveries that led to nuclear weapons.
In October of 1939, shortly after Germany invaded Poland at the start of World War II, an ominous letter from Albert Einstein reached President Franklin Delano Roosevelt. Composed at the urging of Szilard, the letter reported, “it is conceivable … that extremely powerful bombs of a new type may thus be constructed.” American researchers were not alone in their interest in the topic: German scientists, the letter noted, were also on the case.
Roosevelt responded by setting up a committee to investigate. That step would be the first toward the U.S. effort to build an atomic bomb, the Manhattan Project.
On December 2, 1942, Enrico Fermi, who by then had immigrated to the United States, and 48 colleagues achieved the first controlled, self-sustaining nuclear chain reaction in an experiment with a pile of uranium and graphite at the University of Chicago. Science News Letter would later call it “an event ranking with man’s first prehistoric lighting of a fire.” While the physicists celebrated their success, the possibility of an atomic bomb was closer than ever. “I thought this day would go down as a black day in the history of mankind,” Szilard recalled telling Fermi.

The experiment was a key step in the Manhattan Project. And on July 16, 1945, at about 5:30 a.m., the scientists, led by J. Robert Oppenheimer, detonated the first atomic bomb, in the New Mexico desert — the Trinity test.
It was a striking sight, as physicist Isidor Isaac Rabi recalled in his 1970 book, Science: The Center of Culture. “Suddenly, there was an enormous flash of light, the brightest light I have ever seen or that I think anyone has ever seen. It blasted; it pounced; it bored its way right through you. It was a vision which was seen with more than the eye. It was seen to last forever. You would wish it would stop; although it lasted about two seconds. Finally it was over, diminishing, and we looked toward the place where the bomb had been; there was an enormous ball of fire which grew and grew and it rolled as it grew; it went up into the air, in yellow flashes and into scarlet and green. It looked menacing. It seemed to come toward one. A new thing had just been born; a new control; a new understanding of man, which man had acquired over nature.”
Physicist Kenneth Bainbridge put it more succinctly: “Now we are all sons of bitches,” he said to Oppenheimer in the moments after the test.
The bomb’s construction was motivated by the fear that Germany would obtain it first. But it turned out that the Germans weren’t even close to producing a bomb when Germany surrendered in May 1945. Instead, the United States’ bombs would be used on Japan. On August 6, 1945, the United States dropped an atomic bomb on Hiroshima, followed by another August 9 on Nagasaki. In response, Japan surrendered. More than 100,000 people died as a result of the two attacks, and perhaps as many as 210,000.
“I saw a blinding bluish-white flash from the window. I remember having the sensation of floating in the air,” survivor Setsuko Thurlow recalled in a speech given upon the awarding of the 2017 Nobel Peace Prize to the International Campaign to Abolish Nuclear Weapons. She was 13 years old when the bomb hit Hiroshima. “Thus, with one bomb my beloved city was obliterated. Most of its residents were civilians who were incinerated, vaporized, carbonized.”
Humankind entered a new era, with new dangers to the survival of civilization.
“With nuclear physics, you have something that within 10 years … goes from being this arcane academic research area … to something that bursts on the world stage and completely changes the relationship between science and society,” says Reed.
Spotlight
Mathematician J. Ernest Wilkins Jr. was a Manhattan Project standout despite racism
Black scientist J. Ernest Wilkins Jr. made nuclear physics calculations that helped build an atomic bomb.
In 1949, the Soviet Union set off its first nuclear weapon, kicking off the decades-long nuclear rivalry with the United States that would define the Cold War. And then came a bigger, more dangerous weapon: the hydrogen bomb. Whereas atomic bombs are based on nuclear fission, H-bombs harness nuclear fusion, the melding of atomic nuclei, in conjunction with fission, resulting in much larger blasts. The first H-bomb, detonated by the United States in 1952, was 1,000 times more powerful than the bomb dropped on Hiroshima. Within less than a year, the Soviet Union also tested an H-bomb. The H-bomb had been called a “weapon of genocide” by scientists serving on an advisory committee for the U.S. Atomic Energy Commission, which had previously recommended against developing the technology.
Fears of the devastation that would result from an all-out nuclear war have fed repeated attempts to rein in nuclear weapons stockpiles and tests. Since the signing of the Comprehensive Nuclear Test Ban Treaty in 1996, the United States, Russia and many other countries have a maintained a testing moratorium. However, North Korea tested a nuclear weapon as recently as 2017.

Still, the dangers of nuclear weapons were accompanied by a promising new technology: nuclear power.
In 1948, scientists first demonstrated that a nuclear reactor could harness fission to produce electricity. The X-10 Graphite Reactor at Oak Ridge National Laboratory in Tennessee generated steam that powered an engine, lighting up a small Christmas lightbulb. In 1951, Experimental Breeder Reactor-I at Idaho National Laboratory near Idaho Falls produced the first usable amount of electricity from a nuclear reactor. The world’s first commercial nuclear power plants began to switch on in the mid- and late 1950s. But nuclear disasters dampened enthusiasm for the technology, including the 1979 Three Mile Island accident in Pennsylvania and the 1986 Chernobyl disaster in Ukraine, then part of the Soviet Union. In 2011, the disaster at the Fukushima Daiichi power plant in Japan rekindled society’s smoldering nuclear anxieties. But today, in an era when the effects of climate change are becoming alarming, nuclear power is appealing because it emits no greenhouse gases directly.


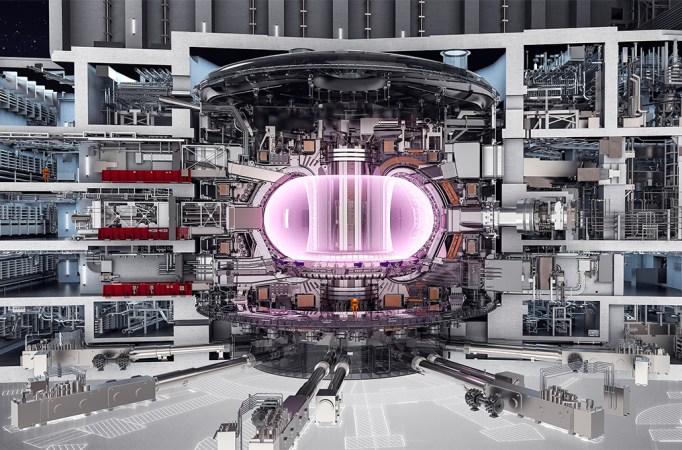
And humankind’s mastery over matter is not yet complete. For decades, scientists have been dreaming of another type of nuclear power, based on fusion, the process that powers the sun. Unlike fission, fusion power wouldn’t produce long-lived nuclear waste. But so far, progress has been slow. The ITER experiment has been in planning since the 1980s. Once constructed in southern France, ITER aims to, for the first time, produce more energy from fusion than is put in. Whether it is successful may help determine the energy outlook for future centuries.
From today’s perspective, the breakneck pace of progress in nuclear and particle physics in less than a century can seem unbelievable. The neutron and positron were both found in laboratories that are small in comparison to today’s, and each discovery was attributed to a single physicist, relatively soon after the particles had been proposed. And the discoveries kicked off frantic developments that seemed to roll in one after another.
Now, finding a new element, discovering a new elementary particle or creating a new type of nuclear reactor can take decades, international collaborations of thousands of scientists, and huge, costly experiments.
As physicists uncover the tricks to understanding and controlling nature, it seems, the next level of secrets becomes increasingly difficult to expose.
— Emily Conover
From the archive
-
Cosmic Rays Disrupt Atomic Hearts
Physicist Carl Anderson’s cloud chamber reveals “a positively charged particle, probably a proton.” Soon, he would discover new positively charged particles: positrons.
-
Conservation of Energy Law Declared Unnecessary
Radioactive decays suggest energy might not be conserved, an issue later solved by the existence of neutrinos.
-
Italian Discovery May Be First of Super-Elements
Enrico Fermi thought that he was seeing the first element beyond uranium, as reported in this article. He was wrong: He was seeing fission, the splitting of atomic nuclei.
-
Neutron May Not Be Real Ultimate Particle of Nature
Two years after its discovery, some scientists still thought the neutron was a combination of an electron and a proton.
-
Atomic Energy Released
A mixed sense of excitement and alarm is palpable in this article detailing the discovery of fission.
-
Atomic Age up to Now
On the first anniversary of the Hiroshima bombing, a timeline of some of the major events reveals a rapid pace of discovery and advancement of the atomic age.
-
Neutrino Now Detected
Scientists report the first signs of lightweight, electrically neutral particles called neutrinos. Full confirmation of their existence would come in 1956.
-
Plethora of Particles
In the 1960s, physicists were flummoxed by the many fundamental particles discovered with accelerators, and the concept of quarks was an attempt to explain their existence.
-
Quark Theory: A Prediction Confirmed
Jets of particles seen in collisions of electrons and positrons clinch the case that quarks exist.
-
Puzzling Search for the GUTs of Physics
Physicists hunt for signs of a Grand Unified Theory, which would unite the three fundamental forces in the standard model, a search that continues today.
-
At Last, Evidence of the Top Quark
After a long search, scientists finally find evidence for the last quark of the standard model, the top quark.
-
Higgs found
Physicists find the last piece of the standard model, the Higgs boson, with the Large Hadron Collider.
-
Emmy Noether changed the face of physics
Many of the past century’s physics advances built on two theorems from mathematician Emmy Noether, as recounted here 100 years after their unveiling.
-
Uncovering Nazi uranium
Two scientists fascinated by the history of nuclear physics unravel the mystery of a uranium cube used in Germany’s failed effort to produce a bomb.
The Latest
-
In a breakthrough experiment, nuclear fusion finally makes more energy than it uses
The sun creates energy through nuclear fusion. Now scientists have too, in a controlled lab experiment, raising hopes for developing clean energy.
-
Antimatter falls like matter, upholding Einstein’s theory of gravity
In a first, scientists dropped antihydrogen atoms and measured how they fell.
-
Here’s how scientists reached nuclear fusion ‘ignition’ for the first time
The first fusion experiment to produce an energy excess required meticulous planning and also revealed a long-predicted heating phenomenon.