All Roads Lead to RUNX
Several autoimmune diseases share one bad actor
“On a good day, it feels like you’re trying to move through a pool of Jell-O.” That’s how Venetia Thompson of Middletown, Del., describes the exhaustion that she’s known most of her life. There have also been periodic headaches and, accompanying each of her three pregnancies, painful lung inflammation. But it wasn’t until she developed a whitening of her fingers 6 years ago, at age 40, that Thompson received a diagnosis of lupus. The whitening is one characteristic of the disease, in which the immune system can attack the skin, joints, lungs, kidneys, blood, and other tissues. Thompson now takes drugs to keep her overactive immune system from running entirely—perhaps fatally—amok.
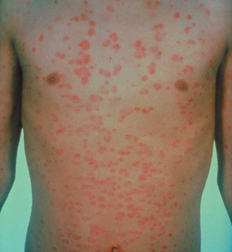
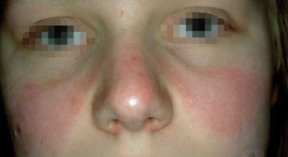
Lupus is just one among scores of recognized autoimmune diseases. In total, the conditions affect an estimated 14 million to 22 million people in the United States. Many of the disorders, including lupus, strike a disproportionate number of women and tend to run in families.
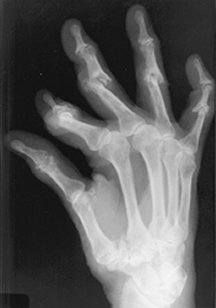
Robbin Baker, 32, of Poulsbo, Wash., was an avid tap dancer and horseback rider before she developed rheumatoid arthritis. Then, bouts of excruciating joint pain in her hands and feet made those pastimes impossible. Baker enjoyed a respite while pregnant
2 years ago, but she can’t fully participate in her daughter’s play activities because symptoms returned after childbirth.
Compared with the primarily internal symptoms of lupus and rheumatoid arthritis, signs associated with psoriasis can be embarrassingly obvious. Since the age of 19, Mark Lemelin, now 46, of Broomfield, Colo., has had nearly every patch of skin on his body covered in scales and rashes at one time or another. Worse than any social discomfort, Lemelin says, are the itching, burning, and stinging that he feels constantly in hundreds of spots. Conscientiously applying the required small amount of topical medications to every inch of psoriatic skin would take more than 2 hours a day, he says.
Scientists are learning that psoriasis, rheumatoid arthritis, and lupus, despite their distinct symptoms, at least sometimes stem from irregular behavior of the same molecule. Three teams of geneticists, each headquartered on a different continent and investigating a different disorder, have recently uncovered genes that seem to be controlled by the same protein. It’s called RUNX1, which stands for runt-related transcription factor 1.
Scientists had previously recognized that RUNX1 acts as a molecular executive with several managerial roles in the immune system. Like other transcription factors, RUNX1 interacts with DNA to enhance or suppress the activity of specific genes. Some of those genes are involved in autoimmunity, the new work indicates.
The findings won’t immediately produce novel drugs or preventive measures for any autoimmune disease, says Marta Alarcón-Riquelme of the University of Uppsala in Sweden. But research efforts on diseases that once seemed only loosely connected may now converge on a common molecular target for therapies, she says.
A lack of tolerance
Responsible for both fending off external threats and stamping out internal malignancies, the immune system is the body’s combined military and police force. New recruits are constantly going through a training regimen in which they learn to recognize and tolerate the body’s own tissues. But like officers that overstep their orders, immune cells sometimes turn against innocent bystanders.
When immune cells clash with cells in the skin of an individual with psoriasis, the skin cells divide unchecked and don’t properly mature, so excess layers of flesh scale and slough off. When immune cells attack joints, rheumatoid arthritis is the outcome. In lupus, which is formally called systemic lupus erythematosus, the immune system can attack multiple organs. In severe cases, both psoriasis and lupus can cause joint inflammation that results in arthritic damage.
Autoimmunity can also affect other tissues. For instance, attacks on insulin-making cells in the pancreas cause type 1 diabetes, and attacks on nerve cells lead to multiple sclerosis.
Years of research on autoimmune diseases haven’t revealed many of the fundamental defects within cells, says Anne Bowcock of the Washington University School of Medicine in St. Louis. Psoriasis researchers, for example, still aren’t certain whether a chemical abnormality in the skin sends immune cells there into a frenzy or the problem is instigated by the immune cells themselves.
Medical researchers have begun to look past the diverse manifestations of autoimmunity to solve biological problems shared by multiple conditions. Research that recently linked a single gene to type 1 diabetes and two autoimmune diseases of the thyroid gland suggests that there is a shared underlying biological flaw (SN: 5/3/03, p. 278: Available to subscribers at Upsetting a Delicate Balance: One gene may underlie various immune diseases). Moreover, a single family can be prone to several autoimmune diseases.
A decade ago, Bowcock and a team of her collaborators set out to identify genetic traits that contribute to psoriasis. The researchers recruited 572 people of European ancestry from 242 families in which some individuals had the disease. In DNA from each volunteer, the scientists looked for genetic markers that vary from person to person.
By noting which volunteers had psoriasis, the researchers computed whether specific markers enhanced or reduced a person’s likelihood of disease. The markers that they used are single-nucleotide variations, or polymorphisms, and so are referred to as SNPs (pronounced snips). In a portion of chromosome 17, the team found a handful of SNPs that predicted a person’s psoriasis risk. After tests on additional SNPs in that DNA region, Bowcock and her colleagues identified two short stretches of DNA in which the presence of certain markers increases psoriasis risk.
So far, the researchers have thoroughly investigated only one of these DNA stretches, and they’ve found that it contains two genes separated by about 1,200 individual DNA building blocks, or nucleotides. Past studies suggested that SLC9A3R1, one of these genes, plays a role in immunity, but the function of the other, NAT9, isn’t known. Bowcock and her colleagues found no psoriasis-associated flaw in either gene.
The researchers then hypothesized that a separate segment of DNA in the two-gene region might affect how one or both of the genes behave and thereby trigger psoriasis in some people.
Enter RUNX1. One SNP associated with psoriasis turned out to determine whether RUNX1 can bind at a location between the two genes. Bowcock and her colleagues reported their results in the December 2003 Nature Genetics.
Past research had established that RUNX1 regulates development of white cells, the principal blood cells of the immune system, and that defects in RUNX1 can lead to leukemia. Defective RUNX1 binding sites could cause developing immune cells to go astray because crucial programming genes—presumably including SLC9A3R1, NAT9, or both—don’t respond as they should to the presence of the regulatory protein, Bowcock says.
RUNX1 shares its DNA-binding properties with two other human proteins, RUNX2 and RUNX3, although these two relatives aren’t abundant in tissues that make immune cells. Similar proteins have been found in animals ranging from mammals to insects, suggesting that the proteins have regulated important genes through millions of years of evolution.
One piece, three puzzles
Bowcock’s team wasn’t the first to link RUNX1 to autoimmunity. A year earlier, scientists led by Alarcón-Riquelme in Sweden had reported a connection between a gene and the systemic autoimmune disease lupus. Although the gene wasn’t SLC9A3R1 or NAT9, the connection revolved around RUNX1.
Then, in the same issue of Nature Genetics in which Bowcock’s study appeared, Japanese researchers reported data linking RUNX1 to a third autoimmune condition, rheumatoid arthritis.
“It feels like more than a coincidence that in three different diseases you would have the same finding,” says Alarcón-Riquelme. It’s as if scientists are assembling a jigsaw puzzle for each autoimmune disease, she says, “and some of the pieces we can put in one puzzle or another.”
On human chromosome 2, RUNX1 fits into a binding site that lies in the middle of a gene called programmed cell death 1, or PDCD1. Alarcón-Riquelme and her colleagues examined that gene because past studies had suggested that it plays a role in lupus and that it regulates how immune cells learn to avoid attacking tissues belonging to the body.
Using methods similar to Bowcock’s, the scientists identified seven SNPs in the stretch of DNA that makes up PDCD1. They analyzed these genetic markers in more than 2,500 people, including members of 443 families that contained at least one person with lupus. African Americans showed no association between lupus and any of the markers, but the team observed a relationship between one of the SNPs and lupus in Mexicans and people of European descent.
This SNP keeps RUNX1 from binding to PDCD1, Alarcón-Riquelme and her team reported in 2002. The researchers suspect that the lack of binding somehow disrupts immune cells’ tolerance of normal body tissues.
The Japanese researchers, led by Ryo Yamada of the Institute of Physical and Chemical Research in Yokohama City, focused on a region of human chromosome 5. They examined 18 SNPs in 830 Japanese people with rheumatoid arthritis and 658 people without the disease. One SNP in a gene called SLC22A4 appeared more frequently in the participants with rheumatoid arthritis than in the healthy participants, the researchers found. That SNP makes RUNX1 bind unusually readily to SLC22A4, thereby suppressing it.
The researchers hypothesized that if altering the RUNX1 binding site in SLC22A4 affects how that gene behaves, so should changing the availability of the protein. Indeed, additional experiments have demonstrated a link between rheumatoid arthritis and a mutation in the gene that produces RUNX1.
The Japanese researchers don’t know whether SLC22A4 is active in the spleen and other organs where immune cells undergo a maturing process or in the joints where rheumatic inflammation occurs.
Notwithstanding such uncertainties, the convergence of the three independent lines of research represents a major advance, Alarcón-Riquelme says. The new findings provide the first evidence that a common molecular cause might underlie autoimmune disorders, she says. In each study, that molecule can explain only a fraction of the cases, so other factors must also be at work.
The three lines of research may lead to animal models that scientists can use to provide further information on autoimmune diseases in people. For example, scientists might manipulate specific genes by adding RUNX1 and thus induce autoimmunity in animals.
Such work could identify targets for drugs across a range of autoimmune disorders and would also offer scientists new ways to test treatments before giving them to people, Alarcón-Riquelme says.
The new studies reveal an unexpected and promising opportunity, says William Cookson of the Wellcome Trust Centre for Human Genetics in Oxford, England.
Because a RUNX1-targeting therapy for one disease may have applications for others, there would be great incentive for making advances, and research in the area could gather momentum, he says.
Cookson suggests the RUNX1 findings could also have implications for allergies, which, while not autoimmune conditions, involve an overactive response to outside stimuli. Both asthma and atopic dermatitis feature misdirected immune reactions, and Cookson is now looking for associations between those conditions and the RUNX1-regulated genes that Alarcón-Riquelme’s and Bowcock’s groups identified.
Other recent studies already suggest that several regions of the genome linked to psoriasis harbor genes implicated in atopic dermatitis. If shared genetic defects explain why both ailments produce skin inflammation, Cookson says, “those avenues might open up therapeutic options for a whole host of skin diseases.”







