Dying before Their Time
Studies of prematurely old mice hint that DNA mutations underlie aging
With one look, you can usually tell whether someone is old or young. Wrinkled skin or smooth. Thinning hair or thick locks. Bifocals or Ray-Bans. These are just a few of the overt clues. Far less obvious are the age-related signs that show up on the molecular level. Ask a geneticist where to look and he may point you to a person’s mitochondria. These rod-shaped residents of an animal cell provide the cell with energy, and each mitochondrion has its own DNA strand, which is distinct from the DNA in the chromosomes that dwell in the cell’s nucleus. With age, this mitochondrial DNA (mtDNA) becomes riddled with mutations, both subtle and severe.
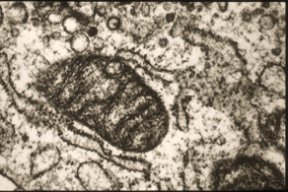
This observation, made in many species, has prompted some researchers to conjecture that the increasing burden of mutations in mitochondrial DNA is a cause of aging. The majority of researchers have thought otherwise. They argue that the mutations are molecular consequences of aging, akin to wrinkles and graying hair.
“You get more gray hair as you get older, but nobody thinks aging is caused by gray hair,” says Nils-Göran Larsson of Karolinska University Hospital in Sweden.
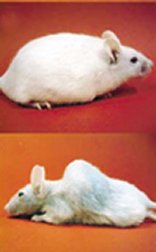
Recently, however, Larsson and his colleagues have genetically engineered mice to develop mtDNA mutations faster than normal. Compared to typical lab mice, these rodents suffer age-related conditions, such as hair loss, osteoporosis, anemia, and infertility, much sooner than typical mice do, and they die young. Many scientists hailed these results, reported in the May 27 Nature, as a vindication of the mitochondrial theory of aging.
“We have been waiting for many years for experimental evidence like this. . . . It is a fundamental advance in aging research,” says David Samuels of Virginia Bioinformatics Institute in Blacksburg.
Samuels finds the new report especially credible because several of its authors, including Larsson, have been publicly skeptical about the hypothesis that mtDNA mutations play a leading role in aging. For example, in a commentary in the February 2003 Aging Cell, Howard T. Jacobs of University of Tampere in Finland challenged the evidence implicating mtDNA mutations.
But the prematurely aging rodents have made him rethink his position.
“I must admit that our findings are provocative,” says Jacobs. “The hypothesis that I confidently expected would fall, did not. . . The mtDNA mutation theory of aging emerges from all this with something of a feather in its cap.”
Some scientists aren’t waiting for further confirmation. They’re already exploring ways of countering the potential effects of the decay in mtDNA. A few researchers have even proposed genetically engineering people to make their mtDNA less prone to mutations.
Cellular liaisons
At some early point in the history of life, two microscopic cells—most likely different kinds of bacteria—apparently struck a complex bargain. One provided a safe, comfortable home inside its own cellular membrane. In exchange, the new tenants produced extra energy for the host cell. The enveloped microbes gradually shed their distinct identities and became what now are called mitochondria.
Mitochondria are the power plants of an animal cell. Some energy-hungry cells, such as those in muscles, contain up to a thousand of these remarkable organelles. Instead of making electricity, as conventional power plants do, mitochondria use oxygen and complex sugars in a process known as respiration. The product is an energy-rich molecule, adenosine triphosphate (ATP), which cells use to drive myriad chemical reactions. Without ATP, cells would be powerless and die.
A mitochondrion’s minuscule anatomy incorporates more than 1,000 different proteins, but most of the genes encoding them have moved into the more sheltered environment of the cell’s nucleus. Remaining within the mitochondrion itself, however, is a small loop of DNA harboring 13 assorted genes. It is this DNA that some researchers suspect may hold the keys to aging.
Because the mtDNA isn’t sequestered in the nucleus, it’s exposed to potentially damaging molecules floating around the cell’s cytoplasm. What’s more, mtDNA doesn’t have the benefit of the repair enzymes available to genes in a nucleus.
Also, the very work that mitochondria do may damage their DNA. The act of creating ATP spawns toxic by-products called free radicals that can rip nearby molecules to shreds. That, conceivably, could underlie various aspects of aging.
“All cells depend on mitochondria for energy, and if the mitochondria begin to fail, then you would expect to see a general slowing of activity throughout all the cells of the body,” says Samuels. “And that description fits with aging.”
Another popular theory holds that aging results from the damage that free radicals do to various molecules in a cell. Free radicals arise not only from ATP production but also from other chemical reactions in the cell. Indeed, one camp of researchers considers the mitochondrial theory of aging a spin-off of the free radical hypothesis. These scientists say that mtDNA is simply a target of the free radicals.
Even so, everyone who studies the molecular basis of aging agrees that mtDNA gets more and more scrambled as animals age. Some of the mutations are subtle—a simple change of a single nucleotide, the basic building block of DNA. Others involve gross rearrangements of the mtDNA sequence or deletions of large stretches of the molecule.
“It’s a universal phenomenon,” says Larsson. “You find these mutations in every aging human. You find [them] in mice, rats, and monkeys.”
Life span limits
Highlighting the potential importance of mtDNA mutations, Samuels reports in the May Trends in Genetics that the vulnerability of mtDNA to deletions may constrain the maximum life span of most mammals to 80 to 100 years. Analyzing previously reported mtDNA sequences of 61 mammalian species, he considered the number of DNA repeats, which are nucleotide sequences that occur twice in close proximity along a DNA molecules. Such repeats mark areas of mtDNA that tend to get deleted with age.
Samuels found that the species with the longer life spans generally have the smaller numbers of certain DNA repeats. Therefore, surmises Samuels, “longer-lived species have mtDNA that is less susceptible to mutation.”
Samuels acknowledges that his findings provide at best “circumstantial evidence” for the mitochondrial theory of aging. That’s why he is so impressed by the results of Larsson and Jacobs and their colleagues.
Those researchers put the mitochondrial theory of aging to a direct test by creating mice with a defective gene for the enzyme that copies mtDNA. “This enzyme . . . has the capacity to proofread newly synthesized DNA. If it makes an error, it can go back and insert the right nucleotide,” says Larsson.
His group used genetic engineering to produce mice that have a version of the enzyme that’s sloppier than normal at proofreading. As a result, deletions and other mutations rapidly accumulate in each mitochondrion’s DNA.
At birth, mice with this defective enzyme look and act healthy. Five months into their lives, however, they already seem to be getting old. They start to lose hair and weight and to develop osteoporosis, which leads to a curvature of the spine. Their hearts enlarge, and the mice become anemic and infertile. All of the rodents in Larsson’s study were dead within 14 months; in contrast, a typical lab mouse lives 24 to 36 months.
“These findings strongly support the idea that mutations in mitochondrial DNA can cause at least some features of aging,” George M. Martin and Lawrence A. Loeb, both of University of Washington in Seattle, conclude in a commentary accompanying the Nature report by Larsson’s group.
“It’s a beautiful paper,” adds mitochondria researcher David Clayton of the Howard Hughes Medical Institute in Chevy Chase, Md. “It will be regarded as a classic. Their claim is warranted—that this [experiment] establishes a causative link between mtDNA mutations and aging.”
That said, the new study leaves many questions unanswered. For example, how does an increase in mtDNA mutations cause so many aging-related problems? One possibility is that key cells may die or fail to perform necessary duties as their mitochondria produce less ATP. Another is that deteriorating mitochondria may, as they struggle to make ATP, unleash a flood of extra free radicals that disrupt other cellular functions.
“My hunch is that the higher mutation load results in a higher rate of generation of reactive oxygen species or other toxic by-products of respiration, but that will need to be painstakingly tested,” says Jacobs.
Other researchers caution that although the recent study shows that mtDNA mutations can cause premature aging in genetically altered animals, it doesn’t demonstrate that these mutations occur naturally at a rate that produces aging.
“The real strength of the paper is that it establishes a causal association between mtDNA mutations and accelerated aging, but what it can’t do is tell us precisely how much the natural variation of aging is due to natural variation in mtDNA-mutation rates,” says David Rand of Brown University in Providence, R.I.
Nonetheless, the mice engineered by Larsson and his colleagues should invigorate research into the processes of aging. “They’ve developed a wonderful tool with enormous potential,” says Judd M. Aiken of University of Wisconsin in Madison.
Aiken is interested in studying the muscles of the mutant mice. As people age, their muscles gradually waste away in a phenomenon called sarcopenia (SN: 8/10/96, p. 90: http://www.sciencenews.org/pages/sn_arch/8_10_96/bob1.htm). Aiken’s team has amassed evidence that this deterioration occurs because more and more mitochondria malfunction with age.
Larsson’s team plans to cross the fast-aging rodents with mice genetically engineered to produce increased amounts of an enzyme that mops up free radicals in mitochondria. If the offspring live longer than 14 months, it would solidify the presumed connection between free radicals, mtDNA mutations, and aging.
Larsson envisions drug companies using the prematurely aging mice to test compounds intended for treating or staving off osteoporosis, sarcopenia, and other age-related conditions. “My goal is not to find something to prolong life but to find something to reduce the pathology of aging,” he says.
For others, prolonging life is the primary goal, and they see an opportunity hidden in the mitochondrial theory of aging. Several scientists, most notably Aubrey D.N.J. de Grey of the University of Cambridge in England, have proposed a way to prevent the decay of mtDNA, and thereby perhaps to prevent aging. They suggest transferring copies of mtDNA’s 13 genes into the safer environs of the cell nucleus, where the rest of the mitochondrial genes reside.
“It’s science fiction right now [but] imaginative,” says Martin.
The case for the mitochondrial DNA theory of aging isn’t closed. Some facets of aging, such as cataracts and a weakening immune system, haven’t turned up in the new mutant mice. This implies that there are multiple causes of aging.
Moreover, Martin and Loeb contend that an ultimate test of the mitochondrial theory of aging would be to create mice whose DNA-copying enzyme is more accurate than normal. That should slow the mtDNA-mutation rate and, if the theory holds, lengthen the lives of the rodents.
The Climate Connection
Cold weather might have selected for life-prolonging stretches of DNA
Some scientists are looking among the 46 human chromosomes for genes that promote longevity in people, but they may be looking in the wrong place. Douglas C. Wallace of the University of California, Irvine contends that he and his colleagues have found a potential fountain of youth in mitochondrial DNA (mtDNA), the loops of genetic material in mitochondria, the energy-producing organelles of human cells.
“We’re finding mtDNA variants that allow people to live longer,” says Wallace.
Human mtDNA consists of 13 genes encoding proteins that enable mitochondria to produce the energy-storage molecule adenosine triphosphate (ATP) in a process known as cellular respiration. That process also generates the heat that maintains body temperature. The amount of heat made by mitochondria depends on their efficiency. The better they are at making ATP, the less heat they generate.
Wallace and his colleagues have recently analyzed the mtDNA of more than 1,100 people from around the world and concluded that some of the differences result from adaptations to the local climate. After comparing the mtDNA of people in tropical Africa to that of people in temperate Europe and arctic Siberia, the researchers argue that residents in colder climates have evolved mtDNA that produces less-efficient mitochondria and so generates additional body heat. “Different physiologies are good for different contexts,” notes Wallace.
In the same report, published in the Jan. 9 Science, the researchers assert that certain mtDNA lineages are associated with increased longevity. These mtDNA variants, found among temperate European populations, appear to produce mitochondria that generate more heat than lineages in tropical Africa do, says Wallace. The more heat and the less ATP that mitochondria make, the lower their production of free radicals, the highly toxic molecules implicated in aging. So, in theory, the mtDNA highlighted by Wallace should slow aging.
Wallace still has work to do to convince his colleagues. “The interpretation that certain mtDNA variants produce more heat and are thus evolutionary adaptations to northern climates is viewed with skepticism,” says David Samuels of Virginia Bioinformatics Institute in Blacksburg. The purported connection between certain versions of mtDNA and longevity, he says, is a “very weak association statistically.”
To convince his skeptics, Wallace plans to genetically engineer mice to have mtDNA similar to the human versions that he argues promote longevity. If those animals live longer, some of that skepticism could wither away.