Chill-out device may protect brain during heart attacks
RhinoChill, already approved for use in Europe, passes safety tests, but more study is needed to determine the extent of its benefits
A portable, backpack-sized device that infuses supercooled mist through the nose appears safe in early tests, and may eventually offer a way to help protect patients from neurological damage following a heart attack. Normally, brain tissue starts to die soon after being deprived of oxygen, but research has found that chilling the brain to around 92 degrees can help lessen permanent damage.
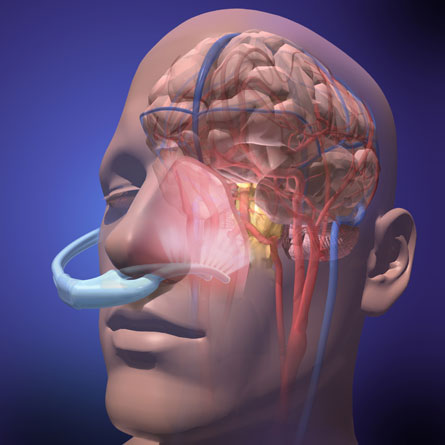
Many hospitals and ambulances already use ice packs, chilled blankets, cold water and other methods to try to lower a patient’s core body temperature after a heart attack. If it holds up to further testing, the experimental machine, called RhinoChill, would provide a way to cool the body quickly and efficiently with a battery-operated technology that does not need refrigeration and can be carried on any ambulance.
“We know it is safe,” said Maaret Castrén, an emergency care researcher at the Karolinska Institute in Stockholm, the study’s lead author. She presented the results November 15 in Orlando during the annual meeting of the American Heart Association’s Scientific Sessions. The study involved 200 patients and was designed to test the safety of the device, which has already been approved for use in Europe, and will go on the market there next year.
Among 182 patients with reported results, about 47 percent of the patients who received cooling survived, compared with 31 percent of those who received only standard care. Of those who survived, 37 percent were considered to be in good neurological condition, as opposed to 21 percent of the comparison group. However, the numbers were too small to rule out the possibility of chance. Larger studies that will gauge effectiveness are planned, Castren said.
The study was sponsored by BeneChill, Inc., the device’s manufacturer.







