Nanosponges sop up toxins and help repair tissues
Tiny particles coated with cell membranes can do more than deliver drugs
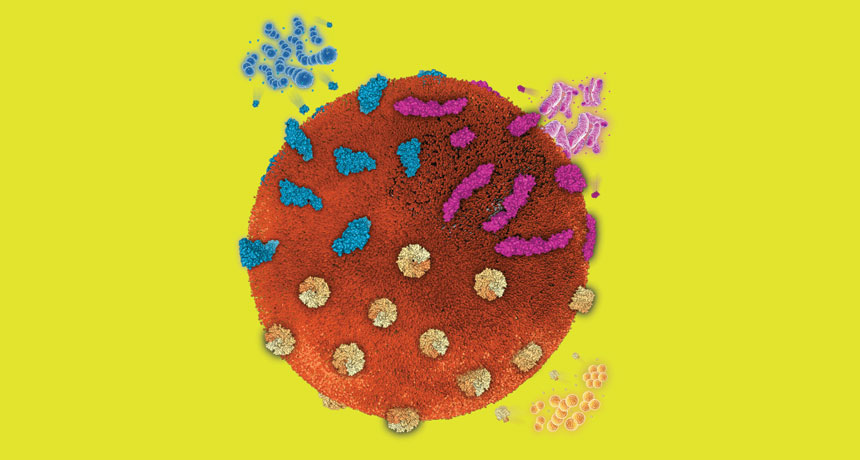
NANOHEALERS A single nanosponge (large sphere in this illustration) can trap toxins produced by a variety of pathogens.
L. Zhang Lab/UC San Diego
To take his fledgling lab to new heights, Liangfang Zhang hatched a plan that he considered brilliant in its simplicity. It involved procedures that many of his peers found a little out there. But if he could make his idea work, it would clear a major hurdle to safely ferry therapies through the body on nanoparticles one-thousandth the width of a human hair.
Yet back in 2010, the young nanoengineer could not convince the National Institutes of Health, the main funder of U.S. biomedical research, to support the project. Zhang applied for funding four or five times over several years, to no avail.
“It felt quite lonely,” he says. “But I just felt this is very unique stuff. And it may become a big thing.”
Pulling funds from other projects and from the start-up package he received to set up his lab at the University of California, San Diego, Zhang did the experiments for his breakthrough paper, published in 2011 in the Proceedings of the National Academy of Sciences. He and coworkers created a new class of nanoparticles, made from carbon-containing polymers, that could slip through blood vessels in a mouse without triggering an immune reaction. While immune responses are important for killing disease-causing pathogens, the same reactions are a nuisance when they clear out molecules made to deliver lifesaving drugs.
Then, instead of just viewing their particles as a drug-delivery system, which most other researchers were focused on, Zhang and his team made a surprising pivot. They repurposed the particles to act as “nanosponges” that trap and remove toxins from the blood. In lab experiments, the nanosponges worked against toxins unleashed by E. coli and some of the harder-to-fight bacteria. Nanosponges also slowed harmful inflammation in mice with a form of rheumatoid arthritis and diverted HIV and Zika from the cells those viruses normally infect, the researchers reported last year.
Nanosponges, which have not yet been tested in people, do their under-the-radar cleanup because of an offbeat idea: The synthetic nanoparticles are coated with membranes from living cells, which helps them blend in. And a single nanosponge can root out a slew of mischief-makers without knowing much about them individually. Many toxins that attack red blood cells, for example, will cling instead to nanoparticles coated with bits of those very cells. Zhang’s team at UC San Diego and others have created a growing arsenal of nanosponges cloaked in membranes of red or white blood cells, each of which absorbs its own set of toxins.
Zhang’s work “opened up the whole field” of membrane-coated nanoparticles, says Omid Farokhzad, a physician-scientist at Brigham and Women’s Hospital in Boston who studies nanoparticles as medicine (SN: 11/11/16, p. 22). Many labs are now “building on the platform that Zhang’s group developed.”
Wild work-around
When Zhang was a postdoc at MIT more than a decade ago, nanoparticle research was all about drug delivery. In 1995, the U.S. Food and Drug Administration approved the first nanotherapy, which carried an anticancer drug. Since then, about 50 other nanodrugs, for treating cancer, hemophilia, multiple sclerosis and other diseases, have made their way into patient care.
Nanoparticles are about one three-thousandth the size of red blood cells, yet each tiny particle can carry thousands of drug molecules. To get a drug where it needs to go, nanoparticles must hide from the immune system. When immune cells encounter foreign material — even nano-sized stuff — the cells try to destroy the invader and remove it from the body. Nanomedicine researchers thought they’d found a solution by coating their particles with a clear, thick liquid called polyethylene glycol, or PEG. Immune cells would “see” the particles as water and not react, Zhang says.
Yet PEG was not ideal. More than a decade ago, patient studies began to reveal that PEG-coated particles can cause people to produce antibodies against PEG, which can trigger unwelcome immune responses. Other approaches, such as outfitting particles with a “don’t eat me” protein that tumor cells use to avoid immune attacks, haven’t worked either. Then Zhang had a flash of insight: “How about disguising the particles as if they belong in the body?”
Trained as a chemical engineer, Zhang was not bound by many biologists’ need to figure out what is going on. “Engineers want to get it done. We want to make it work,” he says. “After making it work, then we may study the reasons.”
The concept of cloaking a human-made particle with bits of unpredictable biological membranes “was very unorthodox” at the time, recalls Che-Ming Jack Hu, the graduate student in Zhang’s lab who took on the project. Today, Hu heads his own nanotechnology lab at Academia Sinica in Taipei City, Taiwan. “People didn’t know what to make of the idea.” But Hu, a bioengineer, found the notion of mixing natural and synthetic materials exciting.
Protect and repair
To keep the immune system off guard, Zhang and Hu set out to disguise the particles in membranes from circulating red blood cells.
Early experiments were nightmarish. “The lab was just setting up. We didn’t have a lot of resources,” Hu recalls. He needed blood from mice, but the group didn’t yet have approval to purchase animals. The researchers turned to neighboring labs. “We’d tell them, ‘If you’re going to kill those mice, can we take some of their blood?’ ” Hu says.
Drawing blood from the animals was not easy for Hu and coworkers, who were used to dealing with chemicals and polymers. “All of a sudden, we were taking these dead mice and sticking a syringe into the heart to take their blood,” he says. At first “we got very little.” Mouse blood, the team learned, clots quickly.
With practice, the researchers were soon collecting a milliliter of blood from each mouse. Hu separated out the red blood cells, stripped their membranes, then nudged the membrane pieces onto nanoparticles.
Each milliliter of blood contained some 5 billion red blood cells, and a cut-up membrane from a single red blood cell could coat several thousand nanoparticles. Injected into live mice, the hybrid particles spread through the body and stayed intact for three days. “We kind of got lucky,” Hu says of those early efforts.
Soon after, a team at the Houston Methodist Research Institute in Texas reported a similar feat, coating nanoparticles with the membranes of white blood cells of the immune system. Like the UC San Diego group, the Houston researchers felt they were going out on a limb by mixing synthetic particles with parts of live cells. “People in the nano field were telling me I was crazy,” says nanoengineer Ennio Tasciotti, the team leader.
The “leukolike” particles could avoid attack by macrophages, which gobble up foreign substances, the researchers showed in a set of experiments published in 2012. The researchers used the particles to carry a cancer drug across a layer of endothelial cells, which make up the lining of blood vessels.
Tasciotti’s team has since replaced synthetic nanoparticles with natural ones, made from thin sheets of fat cells that form spherical blobs called liposomes, the researchers reported in 2016 in Nature Materials. With no artificial parts, Tasciotti says, his leukocyte-coated liposomes, or “leukosomes,” so far tested in lab animals, could face an easier path to FDA approval for use in patients.
Even when the leukosomes weren’t carrying a drug, they interacted with living tissues in helpful ways, Tasciotti says. In 2017 in Nanoscale, he and colleagues reported that leukosomes could relieve inflammation and help repair damaged tissue in the gastrointestinal tract of mice that had a form of inflammatory bowel disease.
Toxin traps
While the Houston team and other labs were tinkering with nanoagents to deliver cancer drugs and heal tissues, Zhang ventured into infectious diseases.
As he and Hu brainstormed uses for their membrane-coated particles, they came upon a key realization. Unlike PEG-coated nanoparticles that just sneak through the body, Zhang and Hu’s nanoparticles are enclosed within biological membranes from cells that normally interact with tissues and with a plethora of chemical messengers and molecules in the body. Perhaps, then, these interactive membrane-coated particles could put up a fight against toxins, the biological weapons of pathogens.
In 2011, as antibiotic resistance was gaining recognition as a serious public health threat, Zhang learned about pore-forming toxins. These small proteins are released by many pathogens, including MRSA, the strain of Staphylococcus aureus that resists most antibiotics. Many of these toxins puncture and kill red blood cells, Zhang says.
Some researchers were taking aim at particular toxins by developing antibodies, specialized proteins produced by immune cells to recognize and bind to specific foreign objects. To Zhang, aiming at one type of toxin at a time seemed tedious. Toxins could come from various sources — MRSA, other bacteria, even animal venom. If a single drug could counteract all the toxins, Zhang says, “that would be really cool.”
Puzzling over this possibility, Zhang and Hu saw new potential in their nanocreations. Rather than merely acting as camouflage for the nanoparticles, the red blood cell membranes might be able to trap toxins. Toxins “like to poke holes in red blood cells,” Hu says. But if toxins go after the nanoparticles, the toxins “get stuck” on the nanoparticles’ membranes and can no longer harm cells. Instead, the troublemakers are carried to the liver and broken down.
Vaccinating mice with red blood cell nanoparticles protected the animals from toxins produced by MRSA and showed potential to protect against toxins from E. coli, poisonous snakes and bees, the UC San Diego team reported in 2013 in Nature Nanotechnology. Zhang, Hu and colleagues have since expanded their collection of nanosponges to include particles covered in membranes from three types of white blood cells — macrophages, neutrophils and T cells.
In a 2017 mouse study in the Proceedings of the National Academy of Sciences, macrophage-coated particles trapped and rendered powerless some of the molecules that drive inflammation leading to sepsis, an uncontrolled response to infection that kills about 6 million people worldwide each year.
Last September, the team reported in Nature Nanotechnology that neutrophil nanosponges sop up toxins that cause rheumatoid arthritis in mice. And in lab dish experiments reported in November in Advanced Materials, nanosponges coated with T cells diverted HIV from actual T cells, the cells this virus typically attacks, eventually causing AIDS.
“It’s a very versatile approach,” says Ankur Singh, a bioengineer at Cornell University. “You don’t have to synthesize a new class of materials each time. You could in principle go with an FDA-approved [particle] and put different cell membranes on the surface.”
But he raises a possible caution with the studies targeting rheumatoid arthritis, an autoimmune disease. Neutrophil membranes often contain proteins called autoantigens, which cause an immune reaction. If such membranes are used to coat nanoparticles, some autoantigens could exacerbate unwanted immune processes that drive rather than fight disease. Further experiments should test diverse patient tissues, Singh suggests.
For now, Cellics Therapeutics, a San Diego–based biotech start-up that Zhang cofounded in 2014, hopes to launch a patient study with the red blood cell nanosponges. Sepsis and pneumonia top the list of about a dozen conditions that could make good targets for the nanosponges.
But the road to FDA approval is uncertain. “It remains challenging to develop even the simplest of nanoparticles,” says Farokhzad at Brigham and Women’s Hospital. Particles with human and synthetic components add “enormously to the complexity of the system,” he says. “But you’re also adding enormously to the promise of the system.”
Today, Hu’s group in Taiwan is taking the nanosponge concept in a new direction. The researchers made nanoparticles coated with membranes from red blood cells and used the particles to trap influenza viruses. The method, reported in 2017 in ACS Applied Materials and Interfaces, might one day improve diagnosis of influenza and other viral infections. “If you think of biological processes — how cells communicate, how viruses infect cells — all these things happen at the nanoscale,” Hu says.
This story appears in the March 16, 2019 issue of Science News with the headline, “Nanohealers: Tiny particles cloaked in cell membranes sop up blood toxins and calm inflammation.”