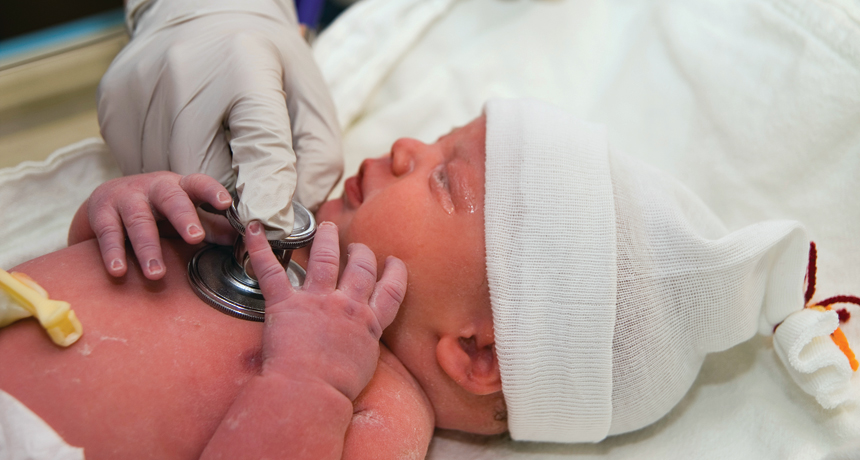
GROWING GOOD BACTERIA An immature form of red blood cell helps suppress the immune system of newborns, experiments with mice suggest. Immune suppression leaves infant mice and humans susceptible to infection but may allow good bacteria to settle in the gut.
KarenMower/iStockphoto







