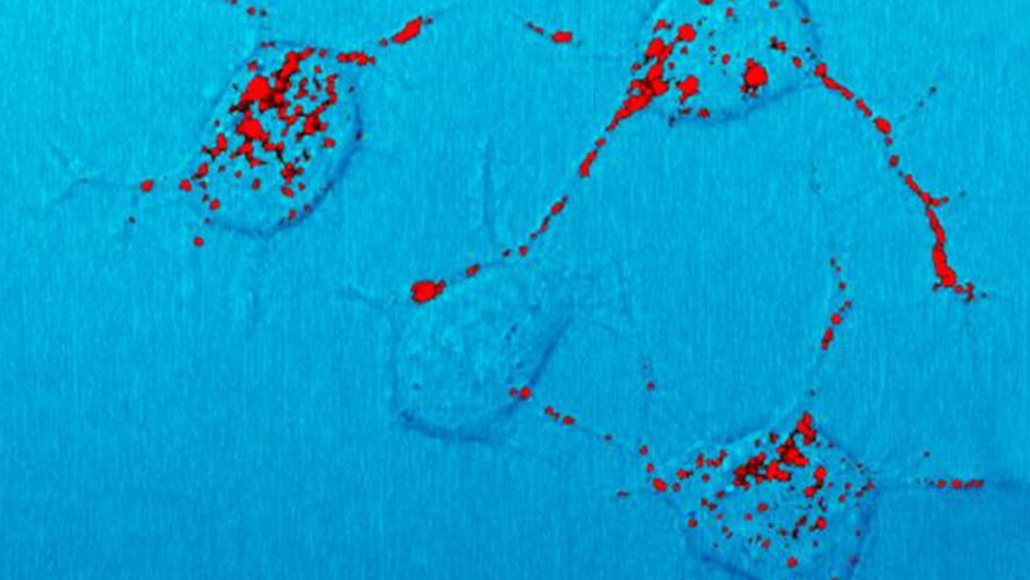
Prion proteins (shown in red in mouse brain cells) can disrupt traffic in the threadlike axons of nerve cells, leading to death of the cells.
NIAID

Prion proteins (shown in red in mouse brain cells) can disrupt traffic in the threadlike axons of nerve cells, leading to death of the cells.
NIAID