What you need to know about coronavirus testing in the U.S.
Testing remains limited in the country
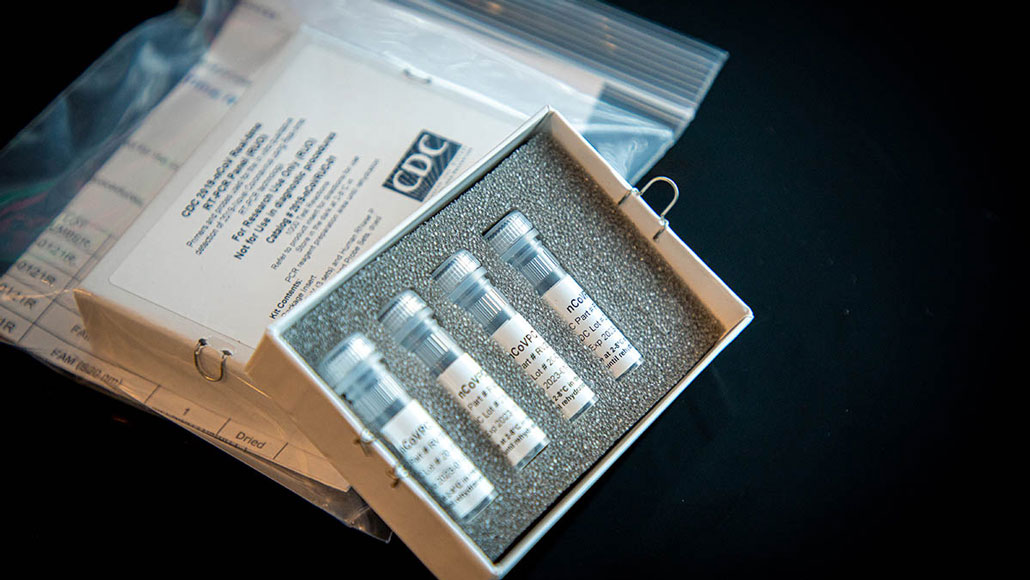
The U.S. Centers for Disease Control and Prevention is shipping its improved SARS-CoV-2 test kit (pictured) to local and state public health laboratories. But government officials warn that there may not be enough tests to keep up with demand.
CDC
U.S. government officials say a million promised tests for diagnosing coronavirus infections will soon be in the mail. But that still leaves many state and local laboratories without the ability to test for the virus, crucial for curbing its spread around the country.
Some states have developed their own tests. Clinical testing companies are now joining the ranks. LabCorp announced March 5 that physicians or other authorized health care providers could already order its test. Quest Diagnostics announced the same day that the company will also offer commercial tests as soon as March 9, pending U.S. Food and Drug Administration reviews. Participation of those two commercial laboratories could greatly expand testing capacity in the United States.
But for now, “we still find ourselves as a country with pretty limited capacity to test,” says Michael Mina, an epidemiologist at the Harvard T.H. Chan School of Public Health in Boston.
Here’s what you need to know about coronavirus testing in the country.
What’s the status of testing?
As of March 11, 81 state and local public health laboratories in 50 states and Washington, D.C., have successfully verified COVID-19 diagnostic tests and are offering testing, according to the U.S. Centers for Disease Control and Prevention. But even states that have tests may have only a single kit, containing enough material to test just 700 people, Mina says.
As of March 13, roughly 14,200 tests have been conducted nationally, according to an analysis from the Atlantic.
That’s up from the 1,583 people that had been tested at CDC, as of March 5. Contrast that with the United Kingdom, where 20,388 people had been tested as of March 6. At that point, only 163 cases of COVID-19 had been detected there.
As of March 13, U.S. health officials have reported over 1,885 coronavirus cases across 47 states and Washington D.C., including 41 deaths. More cases can be expected as testing ramps up, experts say.

Trustworthy journalism comes at a price.
Scientists and journalists share a core belief in questioning, observing and verifying to reach the truth. Science News reports on crucial research and discovery across science disciplines. We need your financial support to make it happen – every contribution makes a difference.
As more cases are found, health officials will need to test contacts of people who carry the virus, and other ill people in affected communities may demand tests, all escalating the need for more tests.
Vice President Mike Pence told reporters March 5, “We don’t have enough tests today to meet what we anticipate will be the demand going forward,” according to CNN. But having companies’ tests in the mix could help testing ramp up.
To get a more complete picture of how widespread the virus is in the United States, “we’re going to need millions and millions and millions of tests,” said Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases in Bethesda, Md., during a CNN town hall on March 5.
How does a test work?
Health professionals will swab a person’s nose or throat, collect phlegm coughed up from the lungs, or squirt liquid into the nose, throat or lungs and collect the liquid again for testing. Neither Quest nor LabCorp will collect such specimens, but doctors or other health providers may send samples to the labs for testing.
Then, those samples are analyzed in a laboratory, where technicians must extract and purify the virus’s genetic material from the mucus, cell debris and other stuff in the samples. “That sample preparation process is usually the biggest bottleneck [in testing],” says Brent C. Satterfield, founder and chief scientific officer of Co-Diagnostics, a company based in Salt Lake City and Gujarat, India, that has developed its own coronavirus test. That test can be used clinically in Europe, but has not yet been approved for use in the United States, although other labs can use components of the company’s test to build their own diagnostic tests.
All of the coronavirus tests being used by public health agencies and private labs around the world start with a technique called polymerase chain reaction, or PCR, which can detect tiny amounts of a virus’s genetic material. SARS-CoV-2, the virus that causes COVID-19, has RNA as its genetic material. That RNA must first be copied into DNA. “That’s a lengthy part of the process, too,” says Satterfield, adding 15 to 30 minutes to the test.
After that, the PCR can begin. The process makes millions to billions of copies of selected segments of DNA. In the case of the coronavirus, the CDC’s original test scanned for three of the virus’s genes, but now tests for two. The World Health Organization’s test, developed by infectious disease researcher Christian Drosten at the Charité – Universitätsmedizin Berlin and colleagues, tests for three genes but is a bit different than the CDC tests. The PCR step typically takes 45 minutes to an hour, Satterfield says.
Some assays give instant yes or no readings, but others may also take time to analyze. All together, it may take about three hours to complete a test, Satterfield estimates.
Can my doctor do the test?
PCR tests are not simple enough to do in a doctor’s office.
In the United States, a doctor is now allowed to decide if a test is warranted and collect the sample, but then must ship the sample off for other trained professionals to prepare and test.
Testing was initially limited to only those people with symptoms and a travel history to an affected area or contact with a known case. On March 4, the CDC relaxed some restrictions on who can get tested. “People still have to be sufficiently sick and have failed a flu test” in order to qualify for coronavirus testing, Mina says.
In some states, the positive test results are called presumptive positives until the CDC can confirm them. In those cases, the final official result may take days. LabCorp estimates that it will take three to four days to return results to physicians.
Why can’t the virus be tested for like influenza?
Many doctors’ offices can do a rapid influenza test. But those flu tests don’t use PCR, Satterfield says. Instead, they detect proteins on the surface of the influenza virus. While the test is quick and cheap, it’s also not nearly as sensitive as PCR in picking up infections, especially early on before the virus has a chance to replicate, he says. By the CDC’s estimates, rapid influenza tests may miss 50 percent to 70 percent of cases that PCR can detect. The low sensitivity can lead to many false negative test results.
Flu tests also aren’t as specific for a particular virus strain as PCR is. About 5 percent to 10 percent of the time, flu tests may mistake a different virus for the flu, creating a false positive result. “Specificity is a big deal when you’re testing large numbers of people who aren’t expected to be positive,” Satterfield says. “If you’re going to test in one of the states that doesn’t have a coronavirus outbreak right now, with a specificity of 90 percent, 10 out of every 100 people are going to show up positive even though the coronavirus isn’t there yet.”
“Accurate diagnosis is a very high imperative for this [coronavirus],” Satterfield says.
An additional benefit of a PCR test is that it may be able to detect viruses earlier in an infection than a flu-style test can, he says, perhaps not in the first day, but a couple of days into an infection when the virus is replicating strongly, but the body’s immune system hasn’t yet begun to fight and produce symptoms. “In every infectious disease I know of, that is the most contagious period for a person; the point in time when the virus has multiplied to its maximum capacity and the body has not yet started to rein in on it,” Satterfield says. Being able to identify people in that period and isolate them from others could help curb the spread of the disease.
Why was broader testing delayed in the first place?
Delays started with a manufacturing flaw in the CDC’s original PCR test, which caused components that detect one of the three targeted viral genes to not work properly, the health agency says.
Those woes sound like user error to Co-Diagnostics’ Satterfield. “A lot of what they are seeing is probably due to inconsistent use in the field,” he says. “Tests that work phenomenally well in the lab, when they are sent to the field, sometimes just don’t work the same,” he says.
Co-Diagnostics’ test also uses PCR but tests for only one gene versus three. “Sometimes the more complexity you have in a test, the more things you have that can go wrong,” Satterfield says.
Some delays in getting testing off the ground came from emergency measures enacted by the FDA, Satterfield says. Normally, big medical testing labs, such as state health labs and companies like LabCorp and Quest Diagnostics, are allowed to develop and validate their own tests. But when the coronavirus was declared a public health emergency on January 31, labs needed “emergency use authorization” before using their tests to diagnose cases. Even the CDC had to get permission to use its test. But on February 29, FDA announced that labs could devise their own tests and use them clinically while waiting for the agency to review their applications. “FDA does not intend to object to the use of these tests for clinical testing while the laboratories are pursuing an EUA,” the agency said in a statement.
“It looks like there were some pretty large blunders that led to some serious delays,” says Mina, the epidemiologist at Harvard. “Instead of reducing the amount of testing at the start of an epidemic … we should have been expanding it as quickly as possible and calling for all hands on deck,” he says.
Those delays and the initial limitations on who could be tested may have allowed some cases to slip through the cracks and start community outbreaks in Washington and California.
Where can I get tested?
It will vary from place to place. If you have symptoms of COVID-19 — fever, dry cough and often fatigue — contact your doctor or local or state health department for more information. Do not go to the emergency room for testing, officials say.
Sign up for our newsletter
We summarize the week's scientific breakthroughs every Thursday.