TRANSPLANT TOLERANCE An emergency liver transplant saved 53-year-old Trent Jackson, but he has struggled to keep his body from rejecting the donated organ.
Matthew Rakola
Trent Jackson’s life changed abruptly in early 2015. The computer engineer thought he had the flu. His then-wife, Donna Sylvia, thought differently. His skin was turning a dark golden yellow, almost brown, “like he was getting some kind of weird tan,” she says. On Wednesday, January 28, Sylvia and Jackson’s brother Todd finally persuaded Jackson to see a doctor.
Sylvia’s suspicions were confirmed: Jackson’s liver had failed. His kidneys shut down, too. Doctors rushed him by air ambulance from Columbia, Md., to Johns Hopkins Hospital in Baltimore. There, he scored 39 on a 40-point scale that gauges how likely a person in liver failure is to die without a liver transplant in the next three months.
People in his condition are often considered too sick for surgery, Jackson says. But on February 15, he got a new liver. “I guess they decided that other than being mostly dead, I was pretty healthy.”
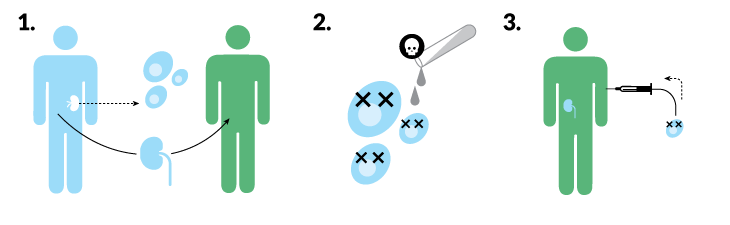
Jackson, 53, got a second chance, but his ordeal hasn’t ended. He takes three drugs every day to keep his immune system from attacking the donor organ. (Transplant recipients often take daunting drug regimens, but many are able to gradually reduce the amount of medication.) Over the long haul, the drugs leave people vulnerable to infections, kidney damage, cancer and type 2 diabetes. Jackson hasn’t experienced the most dire side effects. But tacrolimus, an immunosuppressive drug, makes his hands shake, and a steroid he takes caused cataracts, for which he needed surgery last year.
Powerful as the drugs are, they are not a foolproof rejection remedy. In the three years since his transplant, Jackson has been hospitalized twice for acute rejection. With gallows humor, Jackson, now living in Carrsville, Va., jokes: “The good news is I have a great immune system. The bad news is it tries to kill me every day now.”
Jackson isn’t alone. In the first eight months of 2018, 24,214 people in the United States received a donated organ. In total, there are more than 354,000 people living in the United States with transplanted organs, most of whom are resigned to taking immunosuppressive drugs for the rest of their lives.
Before the drugs, people who got transplants often died within a year, says Andrew Cameron, a transplant surgeon at Johns Hopkins. After the introduction of the drug cyclosporine in 1983, about 80 to 85 percent of transplant recipients survived the first year. That number hasn’t changed much in the last 40 years, Cameron says.
Long-term survival is the bigger challenge. Of 1,456 U.S. lung transplants in 2007, 1,045 had failed by 2017. On the plus side, about 55 percent of transplanted kidneys, 57 percent of livers and 60 percent of hearts survive a full decade.
Cameron and other researchers are looking for ways to help more transplant patients live healthier and longer, without a lifetime of medications. For now, researchers are still experimenting to teach a patient’s immune system to turn a blind eye to, or even welcome, the foreign organ. Scientists are marrying donors’ and recipients’ immune systems, revving up certain calming immune cells and even making the donor organ look a whole lot more like the patient.
The key to acceptance
This struggle to prevent rejection has been going on since doctors began transplanting internal organs in the 1950s, starting with kidneys. Today, transplants also include livers, hearts, lungs, intestines, pancreases and tissues such as skin, bones and tendons. In 2014, hands and faces became an option. Doctors recently transplanted a penis and scrotum for a U.S. veteran wounded in Afghanistan (SN Online: 4/24/18).
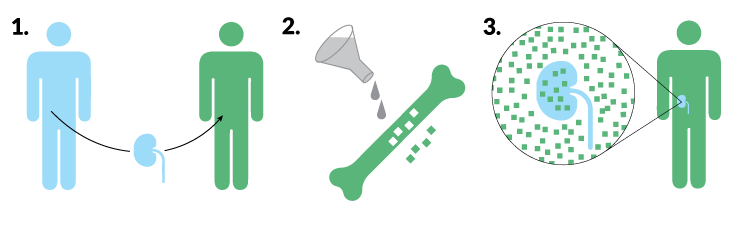
Because transplanted skin has a high likelihood of provoking an immune attack, researchers were skeptical that faces or extremities could be transplanted, Cameron says. But people who have gotten face and hand transplants have needed surprisingly few drugs to minimize rejection. A recipient gets a bit of a donor’s immune system, in the form of blood-producing bone marrow stem cells within the donor’s jaw, hand or arm bones.
Stem cells in the bone marrow give rise to immune system cells that patrol the body and decide what belongs and what doesn’t. Having some of the donor’s immune cells makes the recipient’s body see the donor’s tissue as part of itself (SN Online: 3/7/12). While bone marrow and organ transplant combos are uncommon and still experimental, doctors at Johns Hopkins thought bone marrow infusions after surgery would give the transplanted penis and scrotum the best chance to survive.
Three research groups have developed their own versions of this blended immune system tactic for kidney transplants, with some promising results. The blended immune systems are called “mixed chimeras” for the mythical fire-breathing hybrid with a lion’s head, goat’s body and snake’s tail. Tests in small numbers of patients have been going on for more than a decade at Stanford University, Massachusetts General Hospital in Boston and in a joint venture of the University of Louisville in Kentucky and Northwestern University in Evanston, Ill.
“There have been varying degrees of success,” says John Scandling, a transplant nephrologist at Stanford University Medical Center. “It’s still very much a work in progress.”
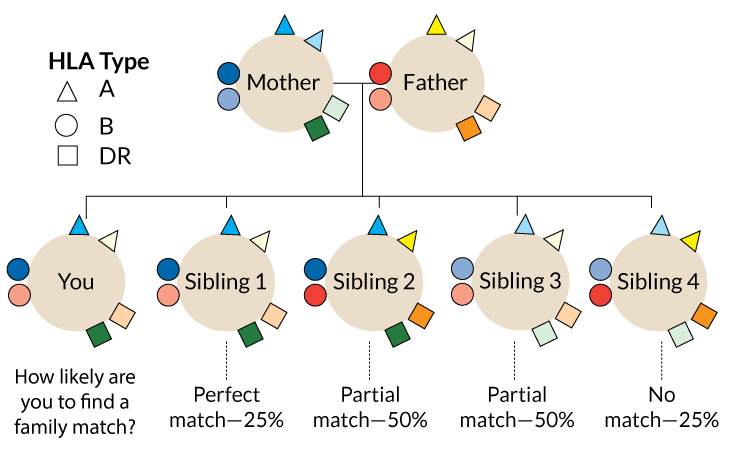
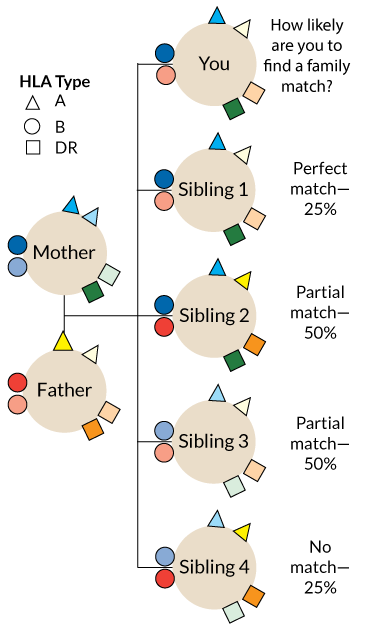
One problem is that the transplanted immune cells don’t always last very long. In 2005, the Stanford group gave 29 patients bone marrow transplants along with kidneys from matched living donors. (Kidneys and livers from live donors last longer than those from deceased donors.) Matched donors and recipients have the same versions of proteins called human leukocyte antigens, or HLAs.
Those proteins help the immune system distinguish cells that belong to the body from invaders, such as viruses, bacteria and other people’s cells. Rejection of a donor organ is more likely the more HLA mismatches a patient has with the donor.
Only nine of the bone marrow recipients still have part-donor immune systems. But even being a temporary chimera can help people hold on to donor organs. Of the 29 patients in the trial, 23 have been able to stop taking immune-suppressing drugs without rejecting the kidney for up to nine years so far, Scandling and colleagues reported in the May Human Immunology. That includes 14 who started with blended immune systems, but eventually lost the donor bone marrow cells.
People who are not well matched to their donors may have a different experience, however. Take Irene Bacani, an accountant in Livermore, Calif., who needed a kidney in late 2013. Her sister, Rezah Burgess, only a partial HLA match, agreed to donate one of hers. Scandling’s team offered the sisters a chance to participate in a Stanford bone marrow stem cell study.
“My sister said right away, ‘Let’s do this,’ ” says Bacani, 53, who was less enthusiastic. Doctors told her she would need radiation to kill some of her bone marrow cells to make room for her sister’s.

Bacani ultimately agreed because there was the tantalizing possibility that perhaps, one day, she wouldn’t need to take immune-suppressing drugs to keep the gift her sister had given her. “I thought, if I don’t try it, I’m always going to be thinking, ‘what if?’ ” On November 30, 2015, Bacani got a kidney from her sister, followed by radiation therapy and then some of her sister’s bone marrow.
Today, less than 2 percent of Bacani’s blood cells come from her sister’s bone marrow. That’s enough to get her down to one daily dose of one drug, tacrolimus, but not enough to go drug free.
Bacani’s story is typical of the mismatched donor-recipient pairs in the Stanford study. None of the HLA-mismatched participants have been able to stop drugs for more than several months before kidney rejection flares up.
A handful of patients in a small study of HLA mismatched kidney transplants at Mass General enjoyed years without immune-suppressing drugs thanks to a transplant of short-lived bone marrow, Hajime Sasaki and colleagues wrote in the May Human Immunology. Seven of 10 patients went off drugs. Three of the seven had to get back on drugs five to eight years after dropping them, either because the kidney disease returned or because of chronic rejection.
Making it stick
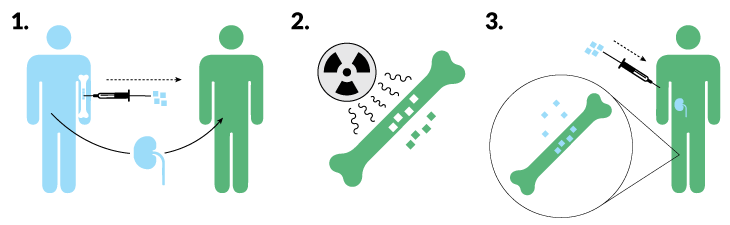
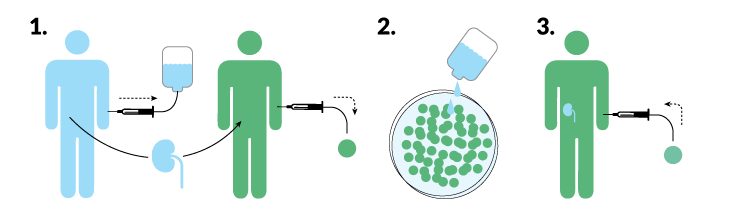
Mixed chimeras work best if the bone marrow stem cells can stick around.
Researchers led by transplant surgeons Suzanne Ildstad of the University of Louisville and Joseph Leventhal of Northwestern may have gotten a handle on that problem. Of 31 patients who got bone marrow and a kidney from a living donor, 23 have kept the donor immune system, and 22 of those have not needed immune-suppressing drugs for eight months to nearly seven years, the researchers reported in May in Human Immunology. Among seven patients who held on to the donor bone marrow for only a little while, five are down to a single low-dose medication, says Ildstad, who is also chief executive officer of the biotech company Regenerex.
Even if the patients can’t completely stop the drugs, reducing the amount of medication is a win in her book.
Ildstad and Leventhal’s secret ingredient is a type of immune cell called a facilitator cell. Regenerex collects facilitator cells from the donor’s blood (using a proprietary procedure) and transplants them with the bone marrow stem cells and the kidney. Those facilitator cells help the donor marrow settle in, so much so that more than 95 percent of the immune cells in the recipient’s blood are made by the donor stem cells. The transplanted immune system ignores the donor kidney, because it looks like home.
But a donor-heavy immune system has its downside. In two of the 31 transplants, the donated immune system began attacking the recipient’s body, a deadly condition known as graft-versus-host disease.
Soothing cells
While those groups are working out the kinks in bone marrow transplants, others are trying to hide the donated organ from immune attack. That’s easier for some organs than others. The liver, in particular, is good at camouflage. When liver transplant patients choose to stop taking their drugs — against all recommendations — about 20 to 50 percent get away with it. (Kidney recipients rarely do, and that kind of freedom is unheard of for heart, lung and pancreas transplants.) Transplant surgeon Sandy Feng of the University of California, San Francisco wanted to know if kids with transplants could go off their meds. She took a look at children with a donated liver who are outwardly healthy but may not be tolerating the organ as well as their doctors think.
In those children, Feng and colleagues have seen what Feng calls “a smoldering rejection process.” All of the 157 children enrolled in Feng’s study had normal liver function according to blood tests. But biopsies revealed that about half of the kids had inflammation or fibrosis, a type of scarring that is a sign of tissue damage, Feng and colleagues report in a study to be published in Gastroenterology.
Feng hopes to smother that rejection with regulatory T cells, or T regs, immune cells that can calm the body’s defenses. In ongoing studies, Feng and colleagues remove a patient’s own T regs and grow them in the lab to expand their numbers. Immune cells called B cells are collected from the donor’s blood or spleen and added to the dish to train the T regs to become familiar with the donor organ so they will quash immune attacks against the organ but not interfere with fighting off infections or cancer.
Trained T regs and other regulatory cells may help donated organs get along with their hosts, but those calming cells are up against a mix of immune cells that want to annihilate the foreign organ. With so many enemies, friendly T regs can only do so much for a transplanted organ, says Xunrong Luo, a transplant immunologist at Duke University. “Just having a ton of good cells is not enough,” she says. “You have to get rid of your bad cells.”
Feng agrees. In patient studies, her group is trying to persuade the body to eliminate the cells that would attack an organ while boosting T regs. The aim is to achieve a balance both the host and the donor organ can live with.
Dead ringers
Luo and colleagues have a different just-ignore-me approach that may allow immune cells to see a donated organ but decide not to attack. The heroes in this technique are dead or dying cells that are perishing via a relatively gentle process called apoptosis, or cell suicide. In animal experiments, Luo’s group takes cells from a donor’s spleen and chemically persuades them to commit apoptosis, then injects the apoptotic cells into the transplant recipient. The apoptotic cells trick the immune system into treating the donor organ as a normal part of the body that it doesn’t need to attack.
The researchers haven’t worked out all the details of how the dying cells pull off the trick, but the team has recently discovered that cells responsible for clearing apoptotic cells from the body are involved in the process. Phagocytes grab and engulf apoptotic cells, then send a signal to decrease production of an inflammation-producing chemical called alpha-interferon, Luo and colleagues reported online August 22 in the American Journal of Transplantation. That stifling of the inflammation signal is important for the host to tolerate a transplant, the researchers found.
Apoptotic cells also marshal protective cells to transplanted organs and fend off attacks, Luo’s team learned from heart transplants in mice. And in unpublished experiments, apoptotic cells have helped avert immune attacks in monkey-to-monkey pancreatic islet cell transplants, Luo says. (Those cells make insulin, a hormone that helps control blood sugar levels.) She is now experimenting with pig-to-monkey transplants.
There is some hope the strategy could work for people, too. One small study showed that apoptotic cells could help patients with bone marrow transplants avoid graft-versus-host disease.
A more familiar organ
Instead of trying to avoid detection or make the recipient more like the donor, Cameron and colleagues are doing the opposite, incorporating a patient’s stem cells into the donor organ. The idea was spawned by studies that showed some long-term liver transplant recipients had livers containing both donor and recipient cells. Stem cells from the recipient’s bone marrow had migrated to the donor liver to repair damage. They became liver cells that grew as part of the foreign organ.
Stem cells are homebodies, rarely leaving the bone marrow for repair missions. But Cameron’s group has used stem cell–mobilizing drugs as a sort of fire alarm that prompts the stem cells to evacuate their home and go in search of organs and tissues in distress. Once the cells find an ailing organ, they set up shop. The donor organ essentially becomes a scaffold that supports growth of a new organ made of a patient’s cells.
The technique actually requires a little bit of rejection to draw the stem cells to the right place. But once the stem cells have integrated, the organ becomes the host’s own as far as the immune system is concerned. Rats and miniature pigs have gotten kidney transplants plus stem cell–mobilizing drugs. Those animals lived with transplanted kidneys without any immune suppression, Cameron and colleagues reported in 2016 in the American Journal of Transplantation.
The pigs had both kidneys removed, then received one kidney from another pig. Pigs that got no immunosuppression or other treatment died within about 10 days, Cameron says.
With one stem cell–mobilizing drug, pigs lived a little longer, about 10 days to a month. Animals that got two stem cell–mobilizing drugs “lived three years and were going strong by the time we declared the experiment over,” Cameron says. Those results suggest that a certain number of host stem cells need to integrate into the transplanted organ, but Cameron and colleagues don’t yet know how many.
If such treatments were applied to people, Cameron says, “we would expect that you’d never reject that kidney just as you never reject your left hand, because it’s ‘self.’ ”
He can even imagine a day when transplants aren’t even necessary. People like Trent Jackson, with a failing liver, may be able to repair their own organs with the help of the stem cell–mobilizing drugs, Cameron’s research suggests. He and colleagues dosed Yorkshire pigs with a liver toxin to cause acute liver failure. Untreated pigs died within 91 hours. With stem cell–mobilizing drugs, five of six poisoned pigs survived, the researchers reported in October in Annals of Surgery.
No one knows which, if any, of these approaches will free transplant recipients to live without fear of rejection. None of the techniques has been vetted enough yet, and none has worked for everyone, Luo points out. Each patient may need a different strategy or a combination of rejection-soothing therapies. Researchers need to push ahead on all fronts and not be afraid to explore other strategies, she says: At this stage of research, “we just cannot be … fixated on one idea.”

Jackson would be happy just to get his liver rejection and medication under control so he can get back to work. As a computer engineer, Jackson was proud of being the guy everyone could count on. His colleagues knew they could just pick up the phone and call him to fix their problems. “There’s no way I’m that person now,” he says.
Bouts of rejection give him yellowed eyes, night sweats, nausea and vomiting. Some days he can’t get going until after noon. “I’m unreliable. I know that.” His health has damaged his sense of self. “I was a go-to person. I was always there. Now I can’t do it.”
But he’s feeling better and his liver is now working as it should. The new research may eventually help Jackson and others like him achieve lasting health. He’d like to be the go-to guy again.
This story appears in the October 27, 2018 issue of Science News with the headline, “Transplant Tolerance: How to get replacement parts to be long-lasting.”
.image-mobile { display: none; } @media (max-width: 400px) { .image-mobile { display: block; } .image-desktop { display: none; } }