How to make recyclable plastics out of CO2 to slow climate change
Chemists are manipulating the greenhouse gas to make clothing, mattresses, shoes and more

Clothing company Zara launched a line of dresses made partially from waste CO2 in 2021. Other businesses are following suit.
Zara
It’s morning and you wake on a comfortable foam mattress made partly from greenhouse gas. You pull on a T-shirt and sneakers containing carbon dioxide pulled from factory emissions. After a good run, you stop for a cup of joe and guiltlessly toss the plastic cup in the trash, confident it will fully biodegrade into harmless organic materials. At home, you squeeze shampoo from a bottle that has lived many lifetimes, then slip into a dress fashioned from smokestack emissions. You head to work with a smile, knowing your morning routine has made Earth’s atmosphere a teeny bit carbon cleaner.
Sound like a dream? Hardly. These products are already sold around the world. And others are being developed. They’re part of a growing effort by academia and industry to reduce the damage caused by centuries of human activity that has sent CO2 and other heat-trapping gases into the atmosphere (SN: 3/12/22, p. 16).
The need for action is urgent. In its 2022 report, the United Nations Intergovernmental Panel on Climate Change, or IPCC, stated that rising temperatures have already caused irreversible damage to the planet and increased human death and disease (SN: 5/7/22 & 5/21/22, p. 8). Meanwhile, the amount of CO2 emitted continues to rise. The U.S. Energy Information Administration predicted last year that if current policy and growth trends continue, annual global CO2 emissions could rise from about 34 billion metric tons in 2020 to almost 43 billion by 2050.
Carbon capture and storage, or CCS, is one strategy for mitigating climate change long noted by the IPCC as having “considerable” potential. A technology that has existed since the 1970s, CCS traps CO2 from smokestacks or ambient air and pumps it underground for permanent sequestration. Today, 27 CCS facilities operate around the world — 12 in the United States — storing an estimated 36 million tons of carbon per year, according to the Global CCS Institute. The 2021 Infrastructure Investment and Jobs Act includes $3.5 billion in funding for four additional U.S. direct capture facilities.
But rather than just storing it, the captured carbon could be used to make things. This year for the first time, the IPCC added carbon capture and utilization, or CCU, to its list of options for drawing down atmospheric carbon. CCU captures CO2 and incorporates it into carbon-containing products like cement, jet fuel and the raw materials for making plastics. Still in early stages of development and commercialization, CCU could reduce annual greenhouse gas emissions by 20 billion tons in 2050 — more than half of the world’s global emissions today, the IPCC estimates.
Such recognition was a big victory for a movement that has struggled to emerge from the shadow of its more established cousin, CCS, says chemist and global CCU expert Peter Styring of the University of Sheffield in England. Many CCU-related companies are springing up and collaborating with each other and with governments around the world, he adds.
The potential of CCU is “enormous,” both in terms of its volume and monetary potential, said mechanical engineer Volker Sick at a CCU conference in Brussels in April. Sick, of the University of Michigan in Ann Arbor, directs the Global CO2 Initiative, which promotes CCU as a mainstream climate solution. “We’re not talking about something that’s nice to do but doesn’t move the needle,” he added. “It moves the needle in many, many aspects.”
Awash in plastic
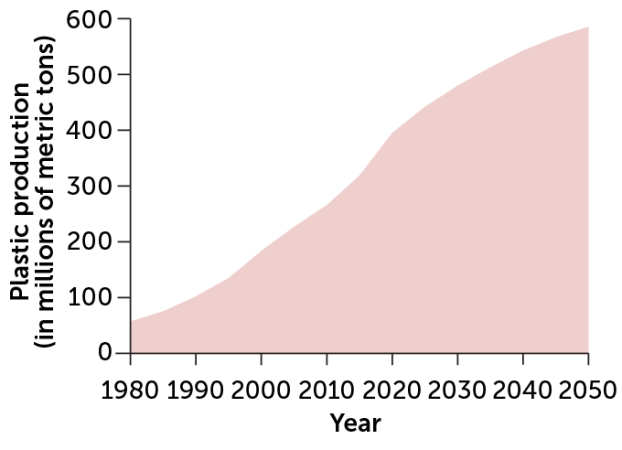
Plastics are made from fossil fuels, and their production is on the rise. Researchers hope to do things differently, using carbon that is already above ground to make plastics.
Global growth in plastic production, 1980–2050
The plastics paradox
The use of carbon dioxide in products is not new. CO2 is used to make soda fizzy, keep foods frozen (as dry ice) and convert ammonia to urea for fertilizer. What’s new is the focus on making products with CO2 as a strategy to slow climate change. Today’s CCU market, estimated at $2 billion, could mushroom to $550 billion by 2040, according to Lux Research, a Boston-based market research firm. Much of this market is driven by adding CO2 to cement — which can improve its properties as well as reduce atmospheric carbon — and to jet fuel, which can lower the industry’s large carbon footprint. CO2-to-plastics is a niche market today, but the field aims to battle two crises at once: climate change and plastic pollution.
Plastics are made from fossil fuels, a mix of hydrocarbons formed by the remains of ancient organisms. Most plastics are produced by refining crude oil, which is then broken down into smaller molecules through a process called cracking. These smaller molecules, known as monomers, are the building blocks of polymers. Monomers such as ethylene, propylene, styrene and others are linked together to form plastics such as polyethylene (detergent bottles, toys, rigid pipes), polypropylene (water bottles, luggage, car parts) and polystyrene (plastic cutlery, CD cases, Styrofoam).
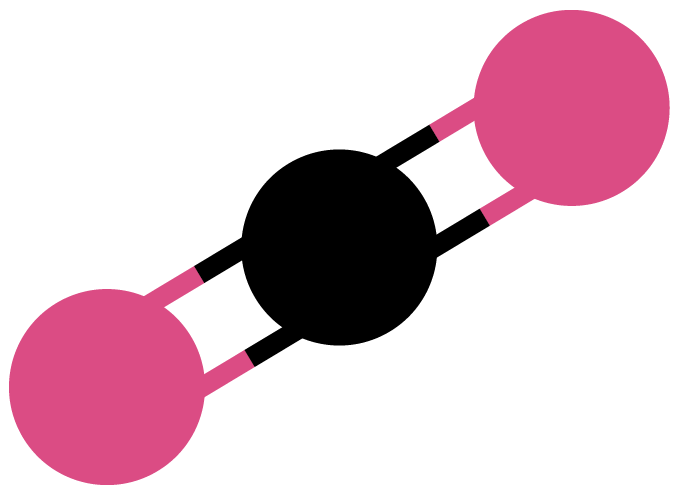
Anatomy of CO2
It takes a lot of energy to break the strong double bonds between the carbon (black) and oxygen atoms (red) in a carbon dioxide molecule. To save energy, researchers are experimenting with chemical and bioinspired catalysts.
But making plastics from fossil fuels is a carbon catastrophe. Each step in the plastics life cycle — extraction, transport, manufacture and disposal — emits massive amounts of greenhouse gases, mostly CO2, according to the Center for International Environmental Law, a nonprofit law firm based in Geneva and Washington, D.C. These emissions alone — more than 850 million tons of greenhouse gases in 2019 — are enough to threaten global climate targets.
And the numbers are about to get much worse. A 2018 report by the Paris-based intergovernmental International Energy Agency projected that global demand for plastics will increase from about 400 million tons in 2020 to nearly 600 million by 2050. Future demand is expected to be concentrated in developing countries and will vastly outstrip global recycling efforts.
Plastics are a serious crisis for the environment, from fossil fuel use to their buildup in landfills and oceans (SN: 1/16/21, p. 4). But we’re a society addicted to plastic and all it gives us — cell phones, computers, comfy Crocs. Is there a way to have our (plastic-wrapped) cake and eat it too?
Yes, says Sick. First, he argues, cap the oil wells. Next, make plastics from aboveground carbon. Today, there are products made of 20 to over 40 percent CO2. Finally, he says, build a circular economy, one that reduces resource use, reuses products, then recycles them into other new products.
“Not only can we eliminate the fossil carbon as a source so that we don’t add to the aboveground carbon budget, but in the process we can also rethink how we make plastics,” Sick says. He suggests they be specifically designed “to live very, very long so that they don’t have to be replaced … or that they decompose in a benign manner.”
But creating plastics from thin air is not easy. CO2 needs to be extracted, from the atmosphere or smokestacks, for example, using specialized equipment. It often needs to be compressed into liquid form and transported, generally through pipelines. Finally, to meet the overall goal of reducing the amount of carbon in the air, the chemical reaction that turns CO2 into the building blocks of plastics must be run with as little extra energy as possible. Keeping energy use low is a special challenge when dealing with the carbon dioxide molecule.
A bond that’s hard to break
There’s a reason that carbon dioxide is such a potent greenhouse gas. It is incredibly stable and can linger in the atmosphere for 300 to 1,000 years. That stability makes CO2 hard to break apart and add to other chemicals. Lots of energy is typically needed for the reaction.
“This is the fundamental energy problem of CO2,” says chemist Ian Tonks of the University of Minnesota in Minneapolis. “Energy is necessary to fix CO2 to plastics. We’re trying to find that energy in creative ways.”
Catalysts offer a possible answer. These substances can increase the rate of a chemical reaction, and thus reduce the need for energy. Scientists in the CO2-to-plastics field have spent more than a decade searching for catalysts that can work at close to room temperature and pressure, and coax CO2 to form a new chemical identity. These efforts fall into two broad categories: chemical and biological conversion.
First attempts
Early experiments focused on adding CO2 to highly reactive monomers like epoxides to facilitate the reaction. Epoxides are three-membered rings composed of one oxygen atom and two carbon atoms. Like a spring under tension, they can easily pop open. In the early 2000s, industrial chemist Christoph Gürtler and chemist Walter Leitner of Aachen University in Germany found a zinc catalyst that allowed them to break open the epoxide ring of polypropylene oxide and combine it with CO2. Following the reaction, the CO2 was joined permanently to the polypropylene molecule and was no longer in gas form — something that is true of all CO2-to-plastic reactions. Their work resulted in one of the first commercial CO2 products — a polyurethane foam containing 20 percent captured CO2. Today, the German company Covestro, where Gürtler now works, sells 5,000 tons of the product annually in mattresses, car interiors, building insulation and sports flooring.
More recent research has focused on other monomers to expand the variety of CO2-based plastics. Butadiene is a hydrocarbon monomer that can be used to make polyester for clothing, carpets, adhesives and other products.
In 2020, chemist James Eagan at the University of Akron in Ohio mixed butadiene and CO2 with a series of catalysts developed at Stanford University. Eagan hoped to create a polyester that is carbon negative, meaning it has a net effect of removing CO2 from the atmosphere, rather than adding it. When he analyzed the contents of one vial, he discovered he had created something even better: a polyester made with 29 percent CO2 that degrades in high pH water into organic materials.

“Chemistry is like cooking,” Eagan says. “We took chocolate chips, flour, eggs, butter, mixed them up, and instead of getting cookies we opened the oven and found a chicken potpie.”
Eagan’s invention has immediate applications in the recycling industry, where machines can often get gummed up from the nondegradable adhesives used in packaging, soda bottle labels and other products. An adhesive that easily breaks down may improve the efficiency of recycling facilities.
Tonks, described by Eagan as a friendly competitor, took Eagan’s patented process a step further. By putting Eagan’s product through one more reaction, Tonks made the polymer fully degradable back to reusable CO2 — a circular carbon economy goal. Tonks created a start-up this year called LoopCO2 to produce a variety of biodegradable plastics.
Microbial help
Researchers have also harnessed microbes to help turn carbon dioxide into useful materials including dress fabric. Some of the planet’s oldest-living microbes emerged at a time when Earth’s atmosphere was rich in carbon dioxide. Known as acetogens and methanogens, the microbes developed simple metabolic pathways that use enzyme catalysts to convert CO2 and carbon monoxide into organic molecules. In the atmosphere, CO will react with oxygen to form CO2. In the last decade, researchers have studied the microbes’ potential to remove these gases from the atmosphere and turn them into useful products.
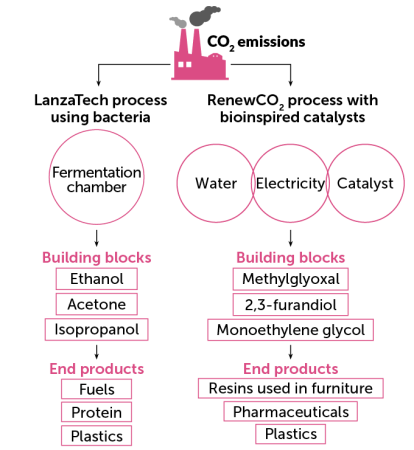
LanzaTech, based in Skokie, Ill., uses the acetogenic bacterium Clostridium autoethanogenum to metabolize CO2and CO emissions into a variety of industrial chemicals, including ethanol. Last year, the clothing company Zara began using LanzaTech’s polyester fabric for a line of dresses.
The ethanol used to create these products comes from LanzaTech’s two commercial facilities in China, the first to transform waste CO, a main emission from steel plants, into ethanol. The ethanol goes through two more steps to become polyester. LanzaTech partnered with steel mills near Beijing and in north-central China, feeding carbon monoxide into LanzaTech’s microbe-filled bioreactor.
Steel production emits almost two tons of CO2 for every ton of steel made. By contrast, a life cycle assessment study found that LanzaTech’s ethanol production process lowered greenhouse gas emissions by approximately 80 percent compared with ethanol made from fossil fuels.
In February, researchers from LanzaTech, Northwestern University in Evanston, Ill., and others reported in Nature Biotechnology that they had genetically modified the Clostridium bacterium to produce acetone and isopropanol, two other fossil fuel–based industrial chemicals. Company CEO Jennifer Holmgren says the only waste product is dead bacteria, which can be used as compost or animal feed.
Other researchers are skipping the living microbes and just using their catalysts. More than a decade ago, chemist Charles Dismukes of Rutgers University in Piscataway, N.J., began looking at acetogens and methanogens as a way to use atmospheric carbon. He was intrigued by their ability to release energy when making carbon building blocks from CO2, a reaction that usually requires energy. He and his team focused on the bacteria’s nickel phosphide catalysts, which are responsible for the energy-releasing carbon reaction.
Dismukes and colleagues developed six electrocatalysts that are able to make monomers at room temperature and pressure using only CO2, water and electricity. The energy-releasing pathway of the nickel phosphide catalysts “lowers the required voltage to run the reaction, which lowers the energy consumption of the process and improves the carbon footprint,” says Karin Calvinho, a former student of Dismukes who is now chief technical officer at RenewCO2, the start-up Dismukes’ team formed in 2018.
RenewCO2 plans to sell its monomers, including monoethylene glycol, to companies that want to reduce their carbon footprint. The group proved its concept works using CO2 brought into the lab. In the future, the company intends to obtain CO2 from biomass, industrial emissions or direct air capture.
Bacteria business
LanzaTech genetically modifies bacteria to transform carbon dioxide into ethanol and other building blocks for plastics and other products. RenewCO2 uses nickel-based catalysts inspired by bacteria to do the same — break down CO2 using little energy to make new things.
Carbon capture with biological conversion
Barriers to change
Yet researchers and companies face challenges in scaling up carbon capture and reuse. Some barriers lurk in the language of regulations written before CCU existed. An example is the U.S. Environmental Protection Agency’s program to provide tax credits to companies that make biofuels. The program is geared toward plant-based fuels like corn and sugarcane. LanzaTech’s approach for making jet fuel doesn’t qualify for credits because bacteria are not plants.
Other barriers are more fundamental. Styring points to the long-standing practice of fossil fuel subsidies, which in 2021 topped $440 billion worldwide. Global government subsidies to the oil and gas industry keep fossil fuel prices artificially low, making it hard for renewables to compete, according to the International Energy Agency. Styring advocates shifting those subsidies toward renewables.
“We try to work on the principle that we recycle carbon and create a circular economy,” he says. “But current legislation is set up to perpetuate a linear economy.”
Doing the carbon math

As companies try to reduce their carbon footprint, many are doing life cycle assessments to quantify the full carbon cost of their products.
The happy morning routine that makes the world carbon cleaner is theoretically possible. It’s just not the way the world works yet. Getting to that circular economy, where the amount of carbon above ground is finite and controlled in a never-ending loop of use and reuse will require change on multiple fronts. Government policy and investment, corporate practices, technological development and human behavior would need to align perfectly and quickly in the interests of the planet.
In the meantime, researchers continue their work on the carbon dioxide molecule.
“I try to plan for the worst-case scenario,” says Eagan, the chemist in Akron. “If legislation is never in place to curb emissions, how do we operate within our capitalist system to generate value in a renewable and responsible way? At the end of the day, we will need new chemistry.”






