Ebola vaccine performs well in U.K. human trial
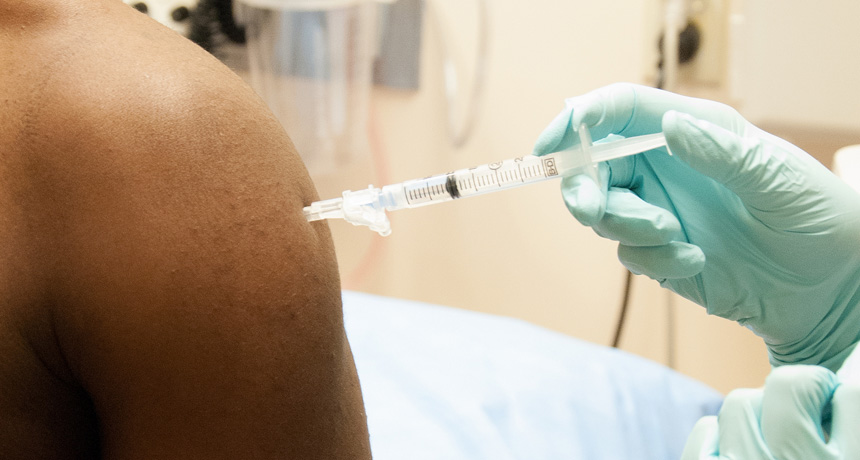
An experimental vaccine against Ebola is relatively safe in humans, a U.K.-based study of 60 people finds. A trial of another vaccine (shown being administered above) produced similar results in 20 patients in a National Institutes of Health study in September.
CC BY 2.0)







