Electrical brain implants may help patients with severe brain injuries
After deep brain stimulation, five patients scored better on an attention test
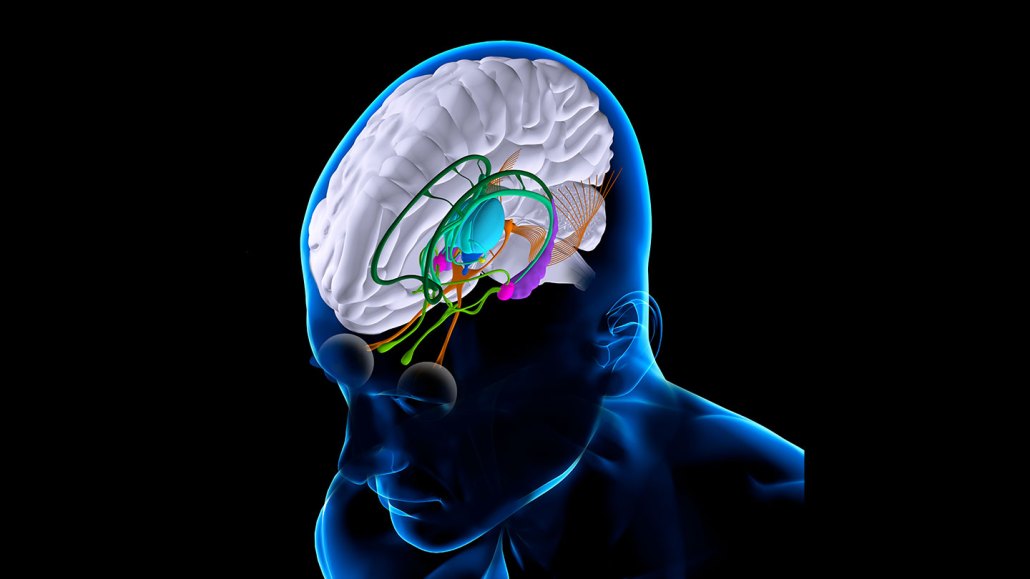
By stimulating part of the thalamus (shown here in turquoise), researchers were able to help patients with traumatic brain injuries regain some lost mental function.
FERNANDO DA CUNHA/Science Source
For people with traumatic brain injuries, cognitive functions like memory, attention and mood regulation can become exceedingly difficult. But “there is no therapy for this kind of problem, even though it’s so prevalent,” says Nicholas Schiff, a neurologist at Weill Cornell Medical College in New York City.
Now, in a small study of individuals who have suffered moderate to severe brain injury, five patients scored better on a test of attention and information processing after having electrodes surgically implanted into the thalamus, an early stop for information coming in through the senses, Schiff and colleagues report December 4 in Nature Medicine. The study participants and their families also reported improvements in their symptoms and daily lives after deep brain stimulation.
The results suggest that direct stimulation of the thalamus could be used to treat cognitive impairment caused by traumatic brain injuries. More than 5 million people in the United States alone live with the effects of moderate to severe traumatic brain injuries, often caused by common events like falls and car crashes.
In deep brain stimulation, electrodes are implanted into the brain and — powered by a pacemaker — used to electrically stimulate targeted brain regions. The technique has long been used successfully to treat other conditions, for instance to quiet the tremors caused by Parkinson’s disease or the seizures of epilepsy. More recently, scientists are studying its ability to treat obsessive-compulsive disorder, eating disorders and deep depression (SN: 9/21/23).
To see if the same approach could restore cognitive function in individuals with traumatic brain injuries, Schiff and colleagues recruited six patients to undergo surgery and have the electrical devices implanted. The time since the patients were injured ranged from two to 18 years ago.
The researchers decided to target the central lateral nucleus of the thalamus, a brain region responsible for relaying information to the brain’s prefrontal and frontal cortexes, which handle executive function. After a severe brain injury, “you have the situation that lots of cells have been disconnected, [and] many cells have died,” Schiff says. Electrically stimulating the relay center of the thalamus, he says, might restore those lost connections.
After identifying the target areas in each person’s brain and implanting the electrodes, the researchers programmed the devices for a 12-hour on/off cycle and optimized them for each patient over a two-week period. One patient developed a scalp infection and had the device removed. The remaining five were put to the test.
The patients were each given a sheet of paper with 25 circles, each containing a number from 1 to 13 or a letter from A to L. The task, called the Trail Making Test, is to draw a line connecting the dots in ascending order while alternating between numbers and letters: 1-A-2-B-3-C and so on.
After receiving stimulation for at least three months, patients decreased the time it took them to connect the circles by about a third on average. One patient, for example, took about 171 seconds to complete the test before treatment, but only about 83 seconds with electrical stimulation. Though the researchers suspected they would see improvement, “our whole group was super gratified and surprised by the effect size,” Schiff says.
In a separate paper published October 18 in Cambridge Quarterly of Healthcare Ethics, the researchers described the participants’ and their families’ thoughts on the treatment. Patients were better able to do everyday things — such as reading, playing video games and watching television — that their injuries had made difficult or impossible. One patient said the treatment made him “more as I was before the accident.” The mother of another told the researchers: “I got my daughter back, I got my daughter back. It’s a miracle.”
Work like this can help patients while also addressing “really fundamental questions about the basic science of human brain function,” says Winston Chiong, a neurologist and ethicist at the University of California, San Francisco who was not involved with the study. Enough is known about potential risks to make treatments reasonable for patients, he says, but there are still many unknowns about the brain.
With deep brain stimulation now shown to be safe and preliminarily effective for treating traumatic brain injuries, Schiff plans to conduct another trial with more patients and for a longer duration. “It’ll be five to 10 times as many patients,” he says, to collect information for an even larger third trial “to try to turn it into therapy.”