To save Appalachia’s endangered mussels, scientists hatched a bold plan
Their rescue effort might just save the river-cleaning mollusks

These mussels got a lot of help to grow large enough to move from a laboratory to a Virginia riverbed.
Gary Peeples/USFWS
The emergency surgery took place in the back of a modified pickup truck in a McDonald’s parking lot in Pikeville, Ky. This scrappy plan to rescue a species of mussel on the edge of extinction made perfect sense: Meet somewhere between Indian Creek in Virginia, where the last known wild golden riffleshells lived, and Kentucky’s Center for Mollusk Conservation in Frankfort, where they would be saved.
The strategy was a malacologist’s version of a Hail Mary pass. One scientist would gingerly pry open three golden riffleshells and remove their larvae to be nurtured in his lab. The other would return the three mussels to Indian Creek, and wait for the day he could introduce their grown offspring to the same habitat. If the plan didn’t produce enough offspring to sustain a new population, the mussels would probably vanish.
Five years ago, Indian Creek was the only known remaining habitat for the golden riffleshell (Epioblasma florentina aureola). And like many other mussels, this bivalve’s future looked bleak. Biologists estimated that only about 100 remained in the wild. “They were the next species on the list for disappearing from the face of the Earth,” says biologist Tim Lane, who leads mussel recovery efforts at the Virginia Department of Wildlife Resources’ Aquatic Wildlife Conservation Center, near Marion. “We were literally watching the last of them.”
Seeing a species vanish in real time is difficult, he says, and is in some ways worsened by the mussels’ near-invisibility beneath the surface. “They’re not charismatic like, say, the northern white rhino,” he says. When mussels go extinct, almost no one knows — or mourns them.

An avid amateur photographer who takes pictures of mollusks, snails, fish and various other small critters in the wild, Lane spends much of his time floating facedown in Appalachian waterways, suspended over rocky riverbeds like a float in the Macy’s Thanksgiving Day Parade. He came up with the plan and carried out phase one: delicately prying the bivalves from the Indian Creek river-bed and laying them in a cooler filled with pebbles, dirt and river water for the 90-minute trip to Kentucky.
A full-grown golden riffleshell is about the size of a small biscuit, with a yellowy, fan-shaped case. Like other mussels, it anchors itself in gravel with a fleshy foot and rarely moves more than a few meters during its lifetime, which could last 15 years or more. The sedentary creatures have been listed as a federally endangered species since 1977.
Malacologists, like Lane and others who study mollusks, are accustomed to championing underdogs. More than two-thirds of all identified North American freshwater mussel species are extinct or endangered. North America has the greatest diversity of freshwater mussels — with a heavy concentration in the Southeast. Tennessee’s Clinch River hosts about twice as many species as all of Europe.
In every locale, the mussels’ problems arise from a mix of factors. Until about a century ago, enormous mussel populations thrived in the Midwest and Southeast, and mussels were often harvested to make shell buttons. But the construction of dams in major rivers divided these populations and separated the creatures from the fish that carry their larvae. “The dams suffocated the huge mussel beds in the most productive habitats,” says Paul Johnson, who runs Alabama’s Aquatic Biodiversity Center, in Perry County.
Adding insult to injury, rampant pollution from industrial dumping and chemical spills led to massive die-offs before the 1972 Clean Water Act led to cleaner waterways. The animals have faced other threats, too, including microbial pathogens and predators.
Just last December, more than 150 kilometers downstream of the confluence of Indian Creek and the Clinch, biologists with the U.S. Fish and Wildlife Service reported a massive die-off of pheasantshells (Actinonaias pectorosa) where the river passes through the town of Kyles Ford, Tenn. The researchers suspect some pathogenic fungi, bacteria or parasites are to blame. Myriad species in Europe and the Pacific Northwest, including the freshwater pearl mussel (Margaritifera margaritifera) and the depressed river mussel (Pseudanodonta complanata), have experienced similar die-offs.
Against that backdrop of known and unknown hazards, researchers around the world are combining in vitro propagation, months of tedious observation and exhaustive laboratory trial and error to save these animals. But none of these evolving methods offer a quick fix.
“It took us 100 years to get into this mess,” Johnson says. “It’s not going to take 10 to get out of it.”

River cleaners
Those who study and try to save mussels feel an irresistible calling, says Jessi DeMartini, a biologist in Illinois who works on mussel conservation in the Forest Preserve District of DuPage County. “It’s an addiction … that becomes a passion.” They see mollusks as the uncelebrated heroes of the world’s rivers.
Mollusk shells stabilize riverbeds and create habitats for other creatures. The bivalves provide food to raccoons, muskrats and other critters. Most importantly, mollusks are nature’s water filters, able to clean up big messes.
A single mussel can filter more than 50 liters of water per day, removing algae and pollution, including toxic substances dumped into rivers as industrial waste. Some researchers suspect that the ability to sop up toxic metals is contributing to the animals’ decline. Like canaries in coal mines, if a mussel population suddenly plummets, it’s a sign that something’s gone foul in the water. (Malacologists describe the smell of a living mussel as rich and sweet, like the river it comes from. But find a dead mussel and the stench is so bad you’d wish you had been born without a sense of smell.)
50+
liters
Amount of water a single mussel can filter in a day
By observing the health of juvenile mussels and analyzing tissue samples, researchers can effectively monitor water quality and acute die-offs, Monte McGregor, director of Kentucky’s Center for Mollusk Conservation, and others reported in December 2019 in Freshwater Science.
The effort to save mussels has implications far beyond the rural and rugged riverways of Appalachia. More than two-thirds of U.S. homes get their drinking water from rivers, Johnson notes. Mussels provide an inexpensive way to safeguard that resource and do some of the work of water treatment plants. “Mussels allow us to provide cleaner water on a less per-cost basis,” he says.
For all these reasons, conservation biologists keep returning to the rivers and take hope where they can find it. The golden riffleshell has been particularly vexing. To even begin the process of mussel propagation, which has a high rate of failure, biologists typically need to start with larvae, also known as glochidia. The golden riffleshell’s dwindling numbers mean that finding a gravid female — one filled with glochidia — is a rare occasion. But on an April morning in 2016, hope came with a find by Sarah Colletti, a mussel-loving biologist also at Virginia’s Aquatic Wildlife Conservation Center. Colletti had joined a small squad of biologists who donned tall rubber waders and spent hours hunched over viewscopes, which look like toy telescopes, pointed down into water to make it easy to tell rocks from mussels. Colletti was scanning the bottom of Indian Creek as part of what’s become an annual ritual, the search for the last remaining golden riffleshells.

It’s a monotonous pursuit, she says, and “you’re second-guessing every rock.” When a mussel comes into view, “it’s kind of shocking.”
Through her viewscope, Colletti spotted three golden riffleshells nestled among the rocks and silt. All were females displaying their lure, a section of tissue that resembles a tasty meal. Those exposed lures meant the mussels were gravid, ready to release millions of glochidia. Finding three gravid females was unusual. The biologists saw an opportunity — maybe one of the last — to help.
Alluring display
Just getting to the larval stage is an accomplishment for these bivalves. Eggs become fertilized only when females filter sperm released into the water by upstream males.
Glochidia, each the size of a grain of salt, can’t survive on their own. They have to clamp onto the gills of a host fish and become parasitic passengers, embedding themselves in the gill tissue and thriving on a mix of nutrients in the water and in fish blood until undergoing a kind of metamorphosis.
As mussels grow their first shells and become juveniles, they swell to the size of a well-fed deer tick, then drop from the fish. For each species of mussel, there’s often only one — or at most, a few — species of fish that can ferry larvae to the next stage of life.
Mussels have evolved a staggering array of methods for infesting fish; almost all involve deception. Some mussels disguise their glochidia in alluring packages that look like minnows; others unspool wormlike appendages tipped with packets holding millions of larvae. The rainbow mussel (Villosa iris) has a lure that looks like a crawfish skittering along the river floor. When a fish tries to eat the minnow or worm or crawfish, the fish gets a mouthful of glochidia, released like dandelion seeds. With the fish’s next gulp of water, the glochidia wash over the gills and stick.

Members of the genus Epioblasma, including the golden riffleshell, have perfected a tactic that earned them the nickname “fish snapper.” The ritual begins when a mother mussel sends out a short thread, the end of which looks like a bug. When a hungry fish swims in for a bite, the shell snaps shut around the fish’s head and holds tight with short, sharp teeth just inside the shell’s rim. As the fish chokes, it inhales the glochidia, which install themselves in the gills. After a few minutes, the mussel relaxes and releases its captive. The fish that survive are stunned; smaller fish (which aren’t good hosts anyway) may die, their heads crushed by the mollusk’s snap.
The handoff
All the pieces of this choreographed sequence — fertilization to glochidia formation to infestation of a host — have to happen in just the right way, says McGregor, who with fellow Kentucky biologist Leroy Koch was waiting at the McDonald’s for Lane to arrive. “There are lots of strikes against these mussels,” he says. “The glochidia have to hit the right fish at the right time.”
Ideally, mussels would reproduce on their own and people wouldn’t have to intervene. Malacologists step in when a species looks like it’s on the brink of extinction.


That morning in April, Colletti marked the location of the mussels in the stream with three large stones and a bright orange flag. She phoned Lane, who had spent much of graduate school studying the diversity of life in Appalachian rivers. The golden riffleshell always seemed to be foundering. In previous years, when they found gravid females in Indian Creek, Lane and colleagues had attempted streamside infestations: catching host fish and manually transferring glochidia from the mussel into the fish gills. But the approach didn’t work.
Lane called McGregor, who was well-known in the close-knit malacology community for having pioneered in vitro approaches to bring bivalves back. Biologists have sent him glochidia in test tubes via UPS and FedEx; he’s also been known to drive for hours to secure the larvae. At Kentucky’s Center for Mollusk Conservation, he closely monitors the temperature and quality of the water that flows through the lab, and he makes his own food for the mussels — often customizing a recipe to fit the needs of a species. After Lane called and proposed the plan, McGregor agreed to meet in Pikeville and carry out the glochidia-removing procedure in what he calls his “mobile lab” (the topped bed of his Ford F-250 super duty crew cab).
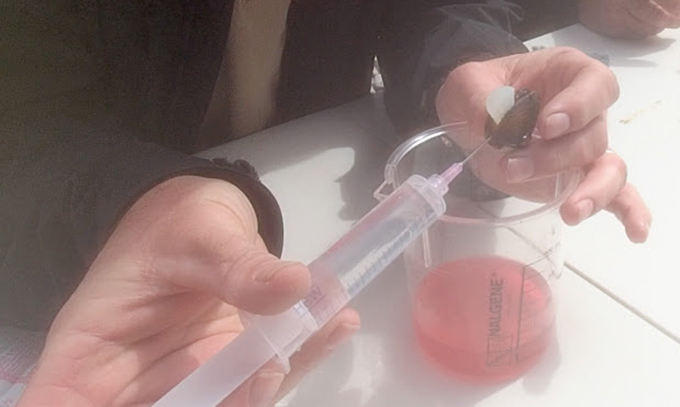
Surgery took no more than 30 minutes per mussel. McGregor pried open the shell about five millimeters with his fingers, and used a silicone wedge to keep it open. Then, he filled a syringe with sterile water and flushed out the glochidia from the mussels into a lab dish. All the while, he had to pay attention to the patient and keep it cool.
“You have to handle the mussel properly,” McGregor says. If the animal gets too warm, that could imperil both the larvae and the mother.
Once the procedure was over, Lane replaced the mussels in the cooler and drove east to return them to Indian Creek. McGregor drove west, escorting thousands of golden rifflleshell larvae over 260 kilometers of twisting mountain roads, to the mussel recovery operation with the longest track record for propagating mussels in the lab without host fish. This would be the golden riffleshell’s best chance at survival.
Take me to the river
For nearly 20 years, researchers at the Kentucky facility have worked on bringing mussels back from the brink of extinction. The small collection of buildings sits near Elkhorn Creek, but McGregor says the water is often too polluted to use for the tanks that hold mussels during the most sensitive part of their development. The pollutants include raw sewage. “We can’t grow mussels in raw sewage,” he says.
If such a thing as “artisanal algae” exists, it’s surely the stuff grown in this lab. Researchers grow algal cultures in giant incubators. McGregor has grown many algal varieties and has spent years matching the right algal slime to the right mussel.

McGregor learned the basics of in vitro propagation in 2004 from Robert Hudson, a malacologist at Presbyterian College in Clinton, S.C. By 2016, McGregor had spent more than a decade improving his recipe, finding the right mix of algae, nutrients and rabbit serum to feed glochidia. Although he prefers to use host fish to grow mussels — and the lab contains dozens of tanks that hold fish as hosts for some other species — scientists have so far been unable to identify the fish that can carry golden riffleshell larvae (which is why streamside infestation doesn’t work).
So McGregor had to grow the larvae without a host. After 18 days in an incubator with McGregor’s custom-made mussel-growing cocktail, about 1,600 larvae survived to become juveniles. They were transferred to silt-lined raceways with cool flowing water to simulate a river. Within a few months, the glochidia had grown to the size of nickels — large enough to survive in the wild.
McGregor divided the spoils. “It was too risky for me to keep them all,” he says. He sent groups of mussels back over the mountains to two facilities in Virginia. One is the Aquatic Wildlife Conservation Center, where Colletti and colleagues have been studying and cultivating the bivalves. In a typical year, researchers there release up to 10,000 lab-grown mussels into the wild, representing up to 10 species.
Colletti says she sees signs of hope for the golden riffleshell. Today, the progeny of those three mussels she found in 2016 are producing their own glochidia in the lab. “They were able to become gravid in captivity,” she says. Lane recently sent photos of those larval grandchildren to McGregor. Colletti and Lane hope the young mussels released into the river will do as well.
There are other, scattered success stories emerging from recent mussel projects. Johnson, in Alabama, has spent years studying the pale lilliput (Toxolasma cylindrellus).
After more than two years of work, Johnson pegged the northern studfish (Fundulus catenatus), which looks like a larger, prettier version of a minnow, as the pale lilliput’s host. Once he made that connection, Johnson began to infest a host fish to cultivate new populations of the endangered species.
There are also big risks. Last year, Johnson propagated about 5,000 juveniles of the rare Louisiana pearshell mussel (Margaritifera hembeli). But just before he was going to release juveniles into a Louisiana river, disaster struck. On an unusually hot spring morning, the temperature of the water streaming into his facility’s raceways soared, killing thousands of the mussels before a researcher could close the valve. “One bad day can literally wreck several years of work,” Johnson says.
He was left with only about 100 animals to return to nature. But those animals have been thriving in the lab. Johnson has grown new batches and plans to restore them to their natural habitat next year. It’s too soon to declare victory, he says, but he’s hopeful.

The ultimate goal in mussel conservation, Johnson says, is to propagate animals that can complete an entire life cycle. That means glochidia get to the host fish, survive the tumultuous juvenile years and mature enough to reproduce. In the wild, the whole process takes a few weeks to a few months. In the lab, the timescale is bigger. “It’s a decadeslong effort,” he says.
Hundreds of the next generation of golden riffleshells are now back at home, with two populations in the Clinch River and one in Indian Creek since 2017. These mussels now measure about the size of a quarter, though some are bigger. Of the 700 that Lane, Colletti and others installed in the wild, many have died and some are unaccounted for, but the researchers estimate that about 300 are still alive.
The scientists placed transponders on about 100 of the mussels, and every year Lane and Colletti return for a census, waving a device that looks like a metal detector over the water surface and waiting for the satisfying chirp that indicates a lab-grown riffleshell is found.
For now, the rescue of the golden riffleshell remains a good news story, but Lane says malacologists have to remain vigilant. “This gives us some time, but it’s not like we can pat ourselves on the back and stop.” To ensure the survival of the species, biologists will need to continue harvesting glochidia, shepherding mussels to the juvenile stage and returning them to the wild, year after year. The ultimate goal is to build a population that can sustain itself and reproduce without human intervention, rabbit serum or emergency surgery outside a rural McDonald’s.






