Videos of gold nanoparticles snapping together show how some crystals grow
The finding could aid in the design of materials whose properties depend on crystal structures
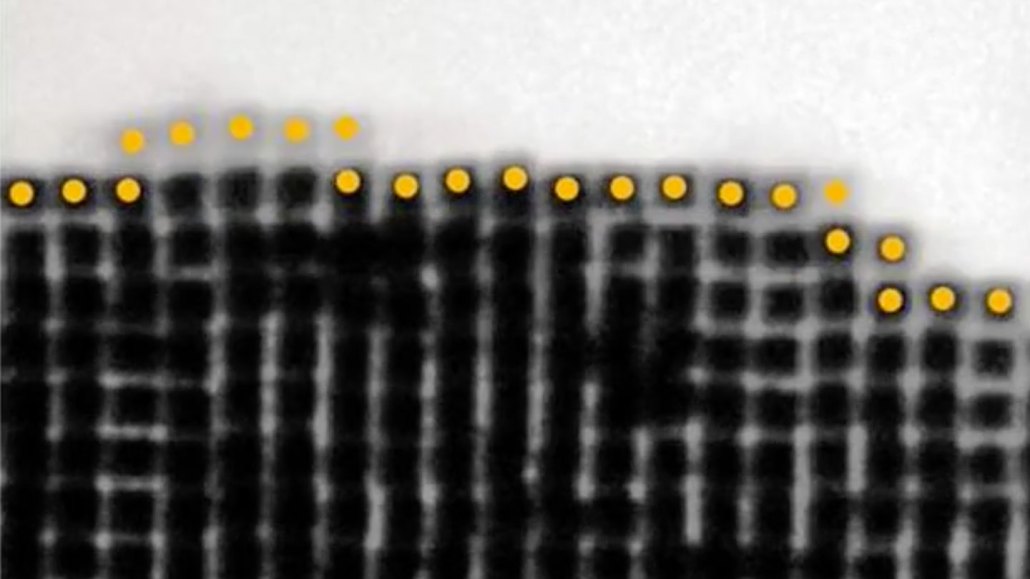
Physicists have now made real-time videos (a still from one shown) of nanoscopic particles locking together into crystals.
Erik Luijten and Qian Chen







