Scientists made human egg cells from skin cells
The proof-of-concept feat could one day help infertile people have children
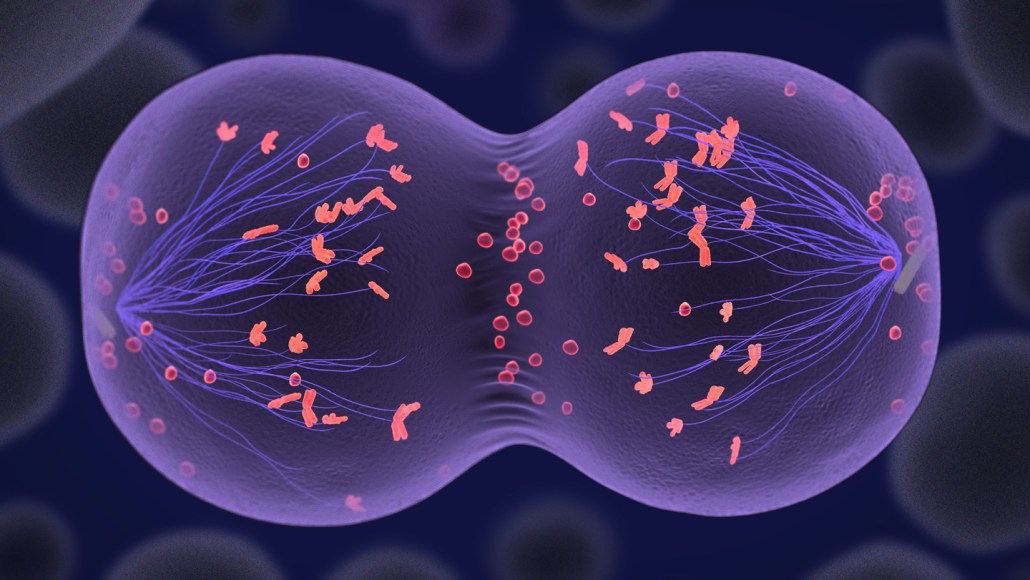
Human skin cells fused with an empty egg were coaxed into expelling half their chromosomes in a process called meiosis (process illustrated here). That allowed them to become functional eggs that could form early embryos.
Tim Vernon/Science Source
Creating human eggs from adult cells just got one step closer to reality.
A technique used in cloning combined with fertilization and a bit of chemical coaxing caused human skin cells to produce eggs able to give rise to early human embryos, researchers report September 30 in Nature Communications.
The effort is the latest attempt to make eggs and sperm from human cells. Researchers have already succeeded in making these important cell types from many types of animals, including pandas. But producing human eggs and sperm has proven elusive.
Such technology may one day treat infertility for women who no longer have eggs because of age, early menopause or previous cancer treatments. Same-sex male couples may also be able to use the technique “to have, potentially, a child that’s genetically related to both partners,” says reproductive endocrinologist Paula Amato of Oregon Health & Science University in Portland.
For now, the technique “is too inefficient and high risk to apply immediately to clinical application,” says stem cell researcher Katsuhiko Hayashi of the University of Osaka in Japan. Hayashi was not involved with this effort but previously reprogrammed tail cells from two adult male mice into eggs and sperm. Those reprogrammed cells gave rise to healthy mice that had two biological fathers and were able to have offspring of their own.
A version of the new technique also works in mice, Amato says. “Usually, things that we can get to work in mice eventually work in humans.” At least, in making stem cells it does.
Amato and colleagues removed the nucleus from a human egg cell and replaced it with the nucleus of a type of skin cell called a fibroblast. That step, called somatic cell nuclear transfer, is the same first step used in cloning Dolly the Sheep and many other species.
But the researchers weren’t trying to make a human clone. They wanted to make an egg cell, which has 23 chromosomes. That’s half the number of chromosomes as most other cells in the body, which carry a set of 23 chromosomes inherited from the mother and 23 from the father.
Cells that will give rise to eggs and sperm go through a type of cell division called meiosis, which halves the number of chromosomes. In that process, each chromosome pairs with its counterpart from the other parent and swaps some DNA. The cell then divides, pulling one half of each pair into daughter cells. Then, when eggs and sperm get together, they produce a zygote with 46 chromosomes that will divide and make every cell in the body.
But the cloned egg already had 46 chromosomes. With mice, Amato and colleagues just fertilized the cloned egg with sperm. That caused the egg to jettison half its chromosomes, producing an embryo with the correct number of chromosomes.
The human eggs, though, didn’t extrude half their chromosomes when fertilized. So the researchers had to add chemical persuasion in the form of a molecule called roscovitine. That molecule allowed the winnowing of chromosomes to begin.
Some of the fertilized eggs made early human embryos, but many did not. “That’s probably, we think, because they had an abnormal number of chromosomes,” Amato says. These failed would-be eggs kicked out half their chromosomes, on average, but not the right half. None of the embryos were allowed to develop beyond the blastocyst stage, growing for about six days. Many stopped developing at earlier stages.
None of the embryos ended up with the correct sets of chromosomes so ultimately were not viable. For instance, one embryo had 48 chromosomes instead of 46. That embryo had all 23 chromosomes from the sperm, but a mishmash of 25 chromosomes from the skin cell. Some chromosomes were present in a single copy, while others had two copies, and other chromosomes were missing entirely.
The unequal divisions probably resulted because the chromosomes paired randomly instead of with their specific mate as they would in normal meiosis, Amato says.
“It was not the outcome we wanted, really, but it was more proof-of-concept. ‘Hey, we can kind of make this process happen now,’” Amato says. The team is working to get the chromosomes to divvy up properly but she estimates at least a decade before the technique could be tested in clinical trials.
Even then, such trials would probably not take place in the United States, which prohibits genetic modification of human embryos, she says.
The new technique has a drawback in that it requires donor egg cells for the cloning step, Hayashi says. Reprogramming as he and colleagues have done does not need egg cells to make other egg cells. Still, he says, “this technology has made a significant breakthrough in halving the human genome.” He predicts “new technologies will stem from this achievement.”







