Invisible ink could be a thing of the past. Tomorrow’s secret codes may instead be hidden in dots of flammable metals. Burning what researchers are calling infofuses releases an encrypted, readable message, representing a new way chemical reactions can be harnessed to store and transmit information, suggests a study appearing online May 26 in Proceedings of the National Academy of Sciences.
“Novelty comes from fresh combinations of old pieces of information,” comments Ehud Keinan of the Technion-Israel Institute of Technology and the Scripps Research Institute in La Jolla, Calif. “This is what we have here: old phenomenon that can be used in a new way.”
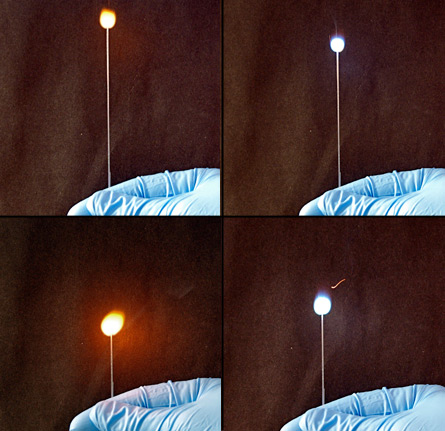
Researchers led by Sam Thomas, a chemist at Harvard University, made the infofuses by printing tiny dots of the flammable alkali metals cesium, lithium and rubidium onto strips of material called nitrocellulose. When sparked, the fuses burn from the top down and send out telltale wavelengths of light each time the flame hits a new spot of metal. “We’re combining chemical sensing with transmission of information,” Thomas says.
Like kids making up a secret code for clandestine communications, Thomas and his team devised a code for each letter in the alphabet, the numbers 1 through 10 and four symbols (., !, ? and @). Two dots in a row — each made from one to three of the metals —represented each symbol. For example, the letter “a” was signified by the burning of a spot of rubidium (no lithium and no cesium), followed by the burning of a spot of cesium (no lithium and no rubidium). And @ was signified by the burning of a dot with lithium, rubidium and cesium followed by a dot of rubidium and cesium (no lithium). The code gave the researchers enough unique signals to accurately send and receive messages, including “LOOK MOM NO ELECTRICITY,” a jab at electricity-dependent LED lights. The only energy source the infofuse needs, Thomas says, is the flick of a lighter.
The alkali metals used in the study emit light at wavelengths indistinguishable to human eyes. Light emissions were read by a spectrometer, a device that measures the exact wavelengths of each pulse of light.
“Although it’s very primitive and simple, it’s a beginning,” Keinan says of the new research. “It will stimulate many new ideas.”
The researchers are next focused on improving the performance of the infofuse, making the fuse longer and the light detectable from farther away. Right now, Thomas says, “You can do sentences, but I don’t know if you want to do War and Peace.”






