Sticky Situations
Scientists are beginning to understand how bacteria find strength in numbers
Every night, a social transformation takes place right under your nose. As you sleep, millions of bacteria in your mouth switch from being free-living drifters to established community members. Those bacteria, which escaped the evening assault of your toothbrush, become part of a sticky coating on your teeth.
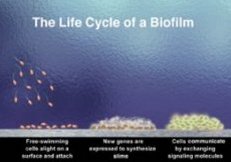
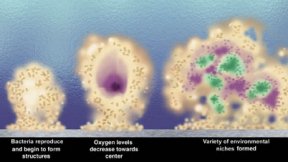
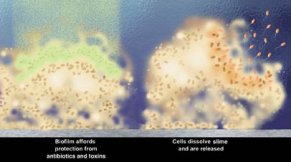

What’s simply annoying for you is a major change in lifestyle for these bacteria and many others.
Bacteria in most environments opt for such communal living at least some of the time. They form colonies called biofilms, which have implications far beyond dental hygiene.
Not only do biofilms coat teeth, but they also form slimes that cover river rocks or foul industrial equipment. The colorful scum around the geysers of Yellowstone National Park consists of biofilms that can be hundreds of years old. Microbial mats in marshes are also long-lasting communities that extend inches into the sand.
Prime real estate for various common bacteria looking for stable homes include contact lenses, intrauterine birth control devices, and surgical sutures. Bacteria that colonize the inner surfaces of medical equipment, such as catheters, are a major source of hospital infections.
Biofilms also are increasingly being implicated in chronic infections. According to estimates from the Centers for Disease Control and Prevention in Atlanta, biofilms account for two-thirds of the bacterial infections that physicians encounter. Many of these are caused by microbes that are common, free-floating inhabitants of the body but become virulent as part of a biofilm community.
Human biofilm infections include dental cavities, gum disease, childhood ear infections, and some infections of the prostate gland and heart. Biofilms also underlie the devastating lung infections that occur in people with cystic fibrosis.
New research is revealing the tremendous changes that bacteria go through, whether on a marsh or a tooth, to become part of an intricate biofilm community.
When bacteria in a biofilm aggregate on surfaces, they produce copious amounts of a sugary, mucous coating. Within this slime, they can form complex communities with intricate architecture featuring columns, water channels, and mushroomlike towers. These structural details may improve nutrient uptake and waste elimination, as blood vessels do in an animal’s body.
In the case of your mouth, teeming bacteria can in just a few hours erect the microscopic equivalent of a coral reef on your teeth.
“We tend to think of bacteria as primitive, single-celled creatures,” says Phil Stewart, who studies bacterial antibiotic resistance at the Center for Biofilm Engineering at Montana State University in Bozeman. “But in biofilms, they differentiate, communicate, cooperate, and deploy collective defenses against antibiotics. Individual microorganisms in a biofilm act together like one multicellular organism.”
Scientific insight into the basis of biofilms is suggesting better ways to vanquish them–with a strategy of divide and conquer.
Prevalence of biofilms
Until recent decades, all knowledge about bacteria came from studies of individual, free-floating cells. Although microscopy pioneer Anton van Leeuwenhoek included biofilm bacteria–conveniently harvested from the plaque on his teeth–among his first observations in the late 1600s, scientists weren’t aware of the complexity and prevalence of biofilm lifestyles until the 1970s.
Now, many scientists argue that the free-floating, or planktonic, lifestyle of bacteria that’s most familiar to laboratory scientists may be nothing more than a way for cells to disperse and colonize new habitats.
Only some of the changes that occur when a bacterium settles down into a biofilm can be observed directly with a microscope. Bacteria have to stick to a surface, aggregate, communicate, and construct their slimy edifices.
To determine the finer steps involved, microbiologists have turned to bacterial genes. Some of these researchers have pieced together relevant parts of the genetics by randomly mutating genes and seeing how biofilms are disrupted.
Karin Sauer of Montana’s Center for Biofilm Engineering takes a different approach. She tracks the biofilm process from start to finish in unaltered bacteria by monitoring the proteins they produce. These indicate what structures or chemical signals the bacteria make at various stages and which genes control them. She presented her early findings about the genetic controls in biofilms last May at the American Society for Microbiology Meeting in Orlando, Fla.
By observing the process without disrupting it, Sauer gets an overview of development, like a parent reviewing snapshots of a growing child, she says.
Her bundle of joy is a soil bacterium called Pseudomonas putida, which uses a long whiplike tail to propel itself through water. This tail, or flagellum, also helps the bacterium to stick to a surface when it first settles down.
In one experiment, Sauer provided her bacteria with hair-thin silicon tubing in which they could make a home. She found that within the first 6 hours, the bacterium turns off genes that make the flagellum.
New equipment
Sauer’s assays indicate that P. putida doesn’t reproduce much during the first several hours. However, it begins synthesizing proteins to make pili, which are multipurpose appendages that look like stiff hairs protruding from a cell’s surface. The bacteria “are suddenly in a new environment, and it’s as if they need all new equipment,” she explains.
The pili can act like Velcro to anchor the bacteria securely to a surface. They can also beat rapidly, enabling the bacteria to swarm over the surface with a twitching movement. Like thousands of tiny tongues or noses, the pili can detect whiffs of chemicals, which may help the bacteria sniff out food or find each other.
Soon after the bacteria start gathering together, they pull out a special set of weapons. They turn on genes to make proteins that help them resist antibiotics. This is when bacteria infecting a person can become nasty.
In her recent experiments, Sauer says, she’s shown for the first time that antibiotic-resistance genes produce more of their defensive proteins in biofilms than in solitary cells.
P. putida normally lives in the soil, where it must fight the onslaught of chemicals that some fungi make to kill off their bacterial competitors. The antibiotic penicillin, made by a mold, is one of the most familiar of these antibacterials.
Some of the bacterium’s resistance genes produce proteins that essentially build a barbed-wire fence around it, says Sauer. Most of these are enzymes that break down antibiotics. Other genes set an internal pump in motion that pushes the hazardous chemicals out of the bacterium as fast as they rush in.
As the bacteria make a simple layer, one cell deep, they begin to produce slime. It protects them from being washed away or drying out and also slows down antibiotics and other toxins that might seep in.
After the first 6 hours, the bacteria start to communicate. They make protein messages and release them, Sauer reports. At first, this appears to be nothing more than chemical chatter.
Then, the chemical messages become so concentrated that whispers turn into shouts. When the messages are loud enough, the bacteria start to pile onto each other, making three-dimensional structures.
After establishing the highly structured biofilm, some of the bacteria turn on their flagella-making genes. Small groups of P. putida then leave the community to start the biofilm-making process over again in another location.
The planktonic and biofilm life stages of P. putida are so different that Sauer compares them to a pair of separate species. It’s “like the difference between a tree and a mushroom,” she says. As many as one-third to one-half of the organism’s genes are used in only one lifestyle or the other, she reports.
Connected to disease
Bacterial biofilms were first connected to human disease and then to antibiotic-resistant infections in the 1980s by Danish pioneers, such as Niels Hiby, and microbiologist William Costerton, who later founded Montana’s Center for Biofilm Engineering.
“What is common to all of these [biofilm infections] is that you can’t easily get rid of the bacteria once they enter the body,” says Michael Givskov of the Technical University of Denmark in Lyngby. The immune system, which can mop up free-floating bacteria in the blood, has difficulty reaching bacteria in biofilms. In most cases, patients’ only defense has been antibiotics, but bacteria in biofilms clearly react differently than lone bacterial cells do to even these assaults.
Sometimes, especially in chronic infections, the biofilm bacteria can gain resistance by the well-known mechanism in which an antibiotic eliminates susceptible cells, and bacteria that happen to become resistant flourish. But physicians also find that bacteria in biofilms often escape harm from antibiotics or other chemicals that kill free-floating cells.
Until recently, most scientists studying biofilms’ drug resistance initially thought that the mucus layer helped to prevent the antibiotic from penetrating. Actually the slime is primarily water, says Stewart, and most antibiotics can penetrate it quite deeply.
New research indicates that a biofilm’s exceptional resistance stems from several characteristics. As they activate specialized resistance genes, bacteria in biofilms seem to benefit from pooling their efforts, Stewart points out. Bacteria can produce an enzyme that inactivates the antiseptic hydrogen peroxide, for example, but a lone cell can’t produce enough of the substance to save itself. A community of bacterial cells, however, can generate a large enough shield of the enzyme to surround and protect the biofilm.
Even when an antibiotic breaches a biofilm’s defenses, the bacteria may be suppressed for only a short time, then flare up again. A large portion of the bacterial cells in a biofilm will be, at any given time, insensitive to a specific antibiotic, says Stewart. “Bacteria in a biofilm occupy a spectrum of physiological states, from rapidly growing to dormant,” he notes. This diversity works to the bacteria’s advantage.
Antibiotics typically target activities like cell division or nutrient uptake in growing bacterial cells. Among active cells in the biofilm, not all succumb to any one antibiotic. Some may be dividing rapidly, while others build membranes or generate energy for the colony.
Dormant cells don’t participate in the activity, so they aren’t usually vulnerable to any of these drugs. Although these bacteria don’t actively contribute to the growing colony, they can weather the catastrophe of antibiotic treatment and quickly renew the biofilm afterward.
The interior of the biofilm also offers a shelter from the antibiotics. It harbors little oxygen, which some antibiotics need to work.
Defeating biofilms
Understanding various aspects of biofilms is leading some researchers to try new ways to defeat them. Instead of escalating the antibiotic arms race with bacteria, Givskov approaches the challenge peacefully. Rather than trying to kill bacteria, he wants to silence them.
Last year, Pradeep K. Singh of the University of Iowa College of Medicine in Iowa City and his colleagues documented chemical communication between bacteria in the lungs of people with cystic fibrosis. Although bacteria enter everyone’s lungs, those of people with this genetic disease can’t sweep the bacteria out, as those of healthy people can. The bacteria form biofilms that can build up large populations, causing life-threatening pneumonia.
Givskov, Hiby, their colleagues in Denmark, and a group in Australia have pioneered the medical use of chemical compounds that target the bacteria’s ability to communicate. Without a call to organize, the researchers reason, the bacteria will remain as loners and be much less likely to cause disease and other problems.
The international team has enlisted the help of a marine alga called Delisea pulchra. It grows off the Australian coast near Sydney in underwater groves full of broad, red-tinged leaves. Films of bacteria coat rocks, dock pilings, and boat bottoms there but not the surfaces of these leaves. The leaves remain free of bacteria, which would block the sun and clog their pores.
D. pulchra doesn’t kill off the bacteria, Givskov finds. It instead produces compounds called furanones that interfere with the bacteria’s cell-to-cell chemical communication.
Furanones isolated from D. pulchra leaves prevent bacteria from forming biofilms in the lab, too.
Givskov and his group have shown that in mice, injected furanones undermine infectious biofilm bacteria. The researchers inoculated mice with bacteria that would infect the animals’ lungs and fluoresce green when they grew there.
Mice that didn’t receive furanones after the inoculation later showed lungs dotted with bright-green bacteria. The lungs of mice injected with furanones soon after being given the bacteria remained dark.
The furanones appear to have prevented the bacteria from organizing themselves well enough to gain a foothold in the lungs, says Givskov.
The experiment illustrates how a compound targeting the bacteria’s organization but not individual cells could have advantages in medicine, he adds. In a cystic fibrosis patient, such a compound could prevent biofilm formation in the lungs without killing bacteria in the patient’s blood. That way, the compound wouldn’t give any resistant bacterial cells an advantage, as antibiotics do, he says.
Unfortunately, the furanones isolated so far aren’t safe to use as a therapy. The team is testing similar compounds and searching for other natural compounds that might work against biofilms without being too harsh.
Furanones, however, may still find uses. Someday, they might keep the bottoms of boats free of damaging bacterial slime. Boaters now use highly toxic, metal-based paints for this purpose.
“The current understanding of biofilm formation is just the tip of the iceberg,” says Givskov. “You have to investigate the phenomenon and find out what’s going on. Then, you can really hit it hard.”
With the current level of research activity, success in the battle against medically and commercially destructive biofilms isn’t far away, Givskov says. He adds, “If it’s possible for nature to do it, why can’t we?”