Worming Your Way to Better Health
To battle autoimmune disease and allergy, scientists tune in to the tricks of parasites
Back in the bad old Stone Age, humans had to put up with all sorts of creepy crawlies. Parasites ran amok in people’s innards, freeloading on nutrient supplies. The parasites took a toll, but over the millennia, those that killed off their meal ticket too quickly didn’t make it. The survivors of this evolutionary shakeout include parasitic roundworms and flatworms, hitchhikers that allow their human host to live on — and to provide three hots and a cot.
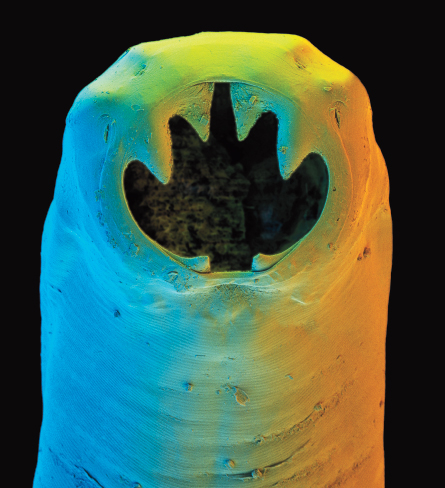
While this scenario might appear to be win-lose, with humans the clear losers, research now suggests that may not be the whole story. In their drive to make humans hospitable hosts, parasites have developed the ability to suppress inflammation aimed against them. And this, it turns out, isn’t necessarily a bad thing.
“They have evolutionarily adapted to this long-standing interaction with their hosts — that’s us — and developed strategies to help the host dampen its immune response,” says Helmut Haas, an immunologist at the Research Center Borstel in Germany.
These strategies are not subtle. But humans have survived the effects and even adapted well to them: A toned-down immunity is, perhaps, the norm. A sober immune system might still defend against enemies while not overreacting to everyday substances in the environment, or otherwise going awry. Suddenly those prehistoric times don’t sound so bad — no Crohn’s disease, no multiple sclerosis, no asthma. Good old Stone Age.
In a stroke of medical inspiration as bold as it is counterintuitive, scientists are now testing this theory by treating patients with live microscopic eggs or larvae of parasitic worms designed to quell these very afflictions. Several clinical research trials are under way and more are planned. Whether promising early results will lead to treatments for these known or suspected autoimmune conditions — and extend to allergy, type 1 diabetes, psoriasis and other cases of immune revolt — remains to be seen.
A marriage on the rocks
Parasitic worms, or helminths, elicit a visceral response from people — in every sense. Worms don’t make for polite dinner conversation and, in industrialized countries, are considered a relic of days thankfully in the past. The notion that helminths are bad guys has been drummed into children of Western nations for several generations, as well as their doctors. (The Latin name for one hookworm is Necator americanus, meaning American killer.)
But helminths have thrived in mammals for millions of years, fine-tuning the parasite act along the way. “These parasites do some harm to the host,” says immunophysiologist Derek McKay of the University of Calgary in Canada. “But if I’m [a parasite] inside a host, at the very least I want the host to reproduce and make more host babies, since my babies will need hosts.”
Besides, an all-out immune war on a helminth might damage the gut. “What we have instead is détente,” says David Elliott, a gastroenterologist at the University of Iowa Carver College of Medicine in Iowa City.
Only recently did the advent of better sanitation and potent drugs break up this marriage. Shoe-wearing put hookworms out of business in the United States. But the 20th century also ushered in asthma, allergy and a host of autoimmune diseases that had been uncommon previously and remain so in less developed countries. All are marked by out-of-control immunity. Vanquishing worm parasites in particular might have disrupted an equilibrium that had served humans well.
Changes in latitudes
Of course, parasitic worms have not really gone away. Some estimates suggest that more than 1 billion people, mainly in the tropics, are infected, giving scientists a chance to study the link between immunity and parasites in context.
In 2003, researchers at the University of Nottingham in England and colleagues found that toddlers living in an Ethiopian city were twice as likely to wheeze — a symptom related to asthma — than were kids living in less sanitary rural areas. Similarly, Ethiopian kids with roundworm infections were half as likely to wheeze as those free of parasitic worms.
At about that time in Argentina, physicians Jorge Correale and Mauricio Farez began a small study that brought the helminth issue into sharp focus. Studies had shown that mice with a disease similar to multiple sclerosis, marked by damage to the fatty sheaths insulating nerves in the central nervous system, improved when infected with parasitic flatworms.
That prompted Correale and Farez to examine a group of MS patients and identify 12, average age 34, who had recently acquired a parasitic worm infection. Then the doctors found 12 other MS patients who matched the first group in age and other respects, but who didn’t have parasites. The rate of MS relapse, or symptom recurrence, had been similar in both groups.
As part of the study, which followed patients for an average of 4.6 years, those harboring parasites agreed not to be treated for them. Perhaps it’s just as well, because MS symptoms in this group became mild to nonexistent. Only three of the patients had a relapse — one apiece — during the study period. The other nine worm-infected patients had none at all. In contrast, the MS patients without parasites had 56 relapses in all, about one per year each. Relapses were treated with prednisone, a steroid drug.
MRI scans, which can detect brain damage that might go unnoticed otherwise, showed 14 brain lesions in the worm-infected patients during the study and 164 in the other group. Mild anemia showed up in four people with worms, but the other eight had no serious ill effects from the parasites. The results appeared in the Annals of Neurology in 2007.
Despite the dramatic difference, the scientists acknowledged that such observational studies need to be borne out in trials in which scientists randomly assign patients to one of two groups with different courses of treatment.
Maria Yazdanbakhsh, an immune-parasitologist at Leiden University in the Netherlands, and colleagues had done just that in Gabon in equatorial Africa. To assess whether a parasitic worm infection protects against allergy, the scientists enlisted 317 schoolchildren, age 5 to 13, in a study. All of the kids had intestinal parasites, mainly roundworms, but none had an active allergy to house-dust mites. Then researchers randomly assigned half the children to get drugs ridding them of the parasites.
Within a year, 14 percent of the children treated for parasites had developed a dust mite allergy, skin-prick tests showed, compared with fewer than 7 percent of those who retained their parasites. The report appeared in the Journal of Infectious Diseases in 2004.
Some people beset by allergies, asthma or autoimmune disease saw these early findings and took to stomping around in less-than-sanitary conditions in Central Africa in hopes of acquiring a parasite to cure ailments. Anecdotal evidence suggests it can work, but the approach is unverified and dangerous. Health risks from worm infections can range from mild (pinworm) to debilitating and sometimes lethal (schistosomiasis).
Others have resorted to buying unregulated parasite eggs on the Internet. “It’s available in this underground market, with mixed results,” says gastroenterologist Jonathan Terdiman of the University of California, San Francisco. He doesn’t endorse the approach, but he does regularly see patients with inflammatory bowel disease even if they are using it. “A lot of these patients are in great need,” Terdiman says. “The pharmaceutical approach has failed them.”
So controlled clinical trials are needed. To get a treatment trusted and cleared, scientists need to test it on people randomly assigned to get the real thing or a placebo. This is the gold standard of medicine and, unlikely as it might have seemed a decade ago, it’s where parasitic worm therapy has now arrived.
Cringe-worthy treatment
Joel Weinstock had a perfectly good career as a parasitologist in the early 1990s when he and Elliott, then colleagues at the University of Iowa, noticed the lack of autoimmune diseases in the tropics. They knew about the hygiene hypothesis, which suggests that early exposure to germs is crucial for normal immune function later on (see “Autoimmunity and the hygiene hypothesis”). And the two knew about the rise of autoimmunity in the West.
“We asked, ‘What’s missing in developed countries?’ ” Elliott says. “We still had viruses and bacteria, but we were missing a whole class — helminths — which used to be universal.” Lab work and tests in animals soon convinced Elliott and Weinstock that helminth infection could quell inflammation. By 2000, they were speculating openly that failure to get parasitic infections might contribute to inflammatory bowel diseases such as Crohn’s disease or ulcerative colitis.
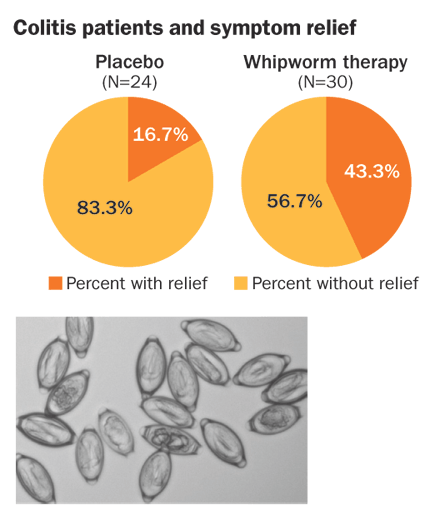
The researchers found an innocuous gut parasite, the pig whipworm Trichuris suis, that didn’t cause disease in people. They asked patients with ulcerative colitis to drink a solution containing either a placebo or cleaned-up, microscopic T. suis eggs every two weeks for 12 weeks. Roughly half the patients were randomly assigned to receive each. Of 30 people getting the helminth therapy, 13 improved substantially, compared with only four of 24 people on the placebo.
Two subsequent studies without placebo comparison groups found that helminth therapy could benefit people with Crohn’s disease. Of 29 Iowa volunteers who got T. suis eggs, 21 went into remission. A British-Australian research team injected hookworm larvae into nine volunteers who had Crohn’s disease. Most showed improvement, marked by less intestinal distress.
Despite the promising findings, helminth therapy isn’t a shoo-in. A placebo-controlled trial in Denmark found no benefit from whipworm eggs given over three weeks for hay fever, scientists reported in January 2010 in the Journal of Allergy and Clinical Immunology. Another trial, at Nottingham, showed that very small doses of hookworm larvae proved no better than a placebo at controlling symptoms in 32 asthma patients. Those data appeared online in December 2009 in Clinical & Experimental Allergy.
But those results haven’t dimmed interest in the strategy. At the University of Wisconsin–Madison, neurologist John Fleming and his colleagues are testing pig whipworm eggs in MS patients, using what tastes like a sports drink to deliver the eggs. At Mount Sinai School of Medicine in New York City, scientists are recruiting participants to test the treatment against autism, which some people hypothesize has autoimmune links. In Boston, researchers at Beth Israel Deaconess Medical Center plan to try it for peanut allergy. A large Crohn’s disease trial is planned for Europe, Weinstock says, and Denmark will host an MS trial. For some trials, the German company Ovamed GmbH is providing live whipworm eggs that are free of bacteria and viruses and are of uniform size.
Purposely ingesting parasitic larvae or eggs “has an audacity about it,” says Fleming. “It doesn’t seem like mainstream science at first pass.”
Not everyone is sold on the approach. “The problem with giving people helminths is you’re introducing a foreign organism, and that has the specter of unforeseen consequences,” McKay says. Though the pig whipworm eggs currently used in clinical trials don’t cause disease in humans, even the idea is, for some, a bit hard to swallow.
The nitty-gritty
Instead of using live organisms, some scientists propose figuring out and mimicking what the parasites do to ratchet down inflammation. The problem is, Fleming says, “Worms know more about the human immune system than we do.”
To catch up, scientists have delved into the molecular mechanisms by which parasites tone down aberrant immune reactions, particularly inflammation. Although inflammation is a normal response to infection that helps in healing, chronic inflammation can damage healthy tissues and plays a central role in most allergy, asthma and autoimmunity.
Weinstock, now at Tufts Medical Center in Boston, links parasites’ effects to changes in innate immunity, the first-line, nonspecific branch of the immune system that reacts against foreign materials or pathogens (in contrast with adaptive immunity in which the body generates agents against specific pathogens). Helminth exposure seems to tilt two innate immune cells, macrophages and dendritic cells, away from promoting inflammation and toward suppressing it, he says.
McKay and his team have studied the effects of helminth exposure by inducing colitis in rodents and then knocking out this inflammatory condition with a tapeworm infection. In response to the infection, the rodents produce more of the immune messenger protein interleukin-10. “We’re very convinced that interleukin-10 is an important part of the anti-inflammatory response driven by worms to block colitis,” he says.
How interleukin-10 got this role is less clear, but McKay thinks it might function as a corrective mechanism, settling down immunity after the body has taken its best shot at killing a parasite. “Maybe interleukin-10 is upregulated to reset the immune system back to normal after the worm is rejected,” he says.
Other researchers are focusing on anti-inflammatory agents of the immune system called regulatory T cells, or T-regs. Run-of-the-mill T cells morph into T-regs when a parasitic infection triggers production of a protein called Foxp3. Also attracting scientific attention: the inflammation-stopping protein TGF-beta and the immune protein interleukin-22.
These cells and proteins could be the active agents knocking back symptoms in people with autoimmunity who have benefited from helminth therapy, Elliott says. And they might all be necessary.
“It appears it’s a lot like getting a kid to clean his room,” he says. “You offer money, turn off the TV, hide the video games — then the room gets cleaned. Any one thing won’t do it.”
The next step is to find out how the parasites induce the host’s cells to make these anti-inflammatory agents.
To that end, Haas of Borstel and his team are studying a nasty helminth called Schistosoma mansoni, which causes schistosomiasis, and focusing on three compounds in the parasite’s eggs that seem to affect only the host. “The worm would not take the effort to make compounds like this, which clearly mediate the interaction with the host immune system, without this being an advantage for them,” Haas says. Sure enough, animal studies show that these parasite compounds ratchet down inflammation.
In Scotland, Rick Maizels and colleagues at the University of Edinburgh have also focused on helminth-secreted compounds, particularly one called cystatin. And other studies in animals point to parasite compounds called glycans. “We’re trying to establish a hierarchy of strength-of-effect,” says Maizels, among parasites and the compounds they unleash to modulate the human immune system.
Some scientists wonder whether helminth therapy (or infection) might even cause permanent changes in immunity.
“Very early imprinting and reprogramming of our immune systems is exciting and possible,” Yazdanbakhsh says. She cites the hygiene hypothesis: “It seems to me that early life events are determining things later.”
Maizels, Haas, McKay and others hope to lay the groundwork for a therapy that tricks the immune system into toning down inflammation, just as the parasites do. “Identifying these [parasite] products could be the blueprints for new drugs,” McKay says.
Yazdanbakhsh predicts the key will be to get precise inflammation-regulating molecules from the parasites. “I tell my students that we don’t want another set of nonspecific immune suppressants, like steroids. We need to be really specific.”
Autoimmunity and the hygiene hypothesis
Genetics would seem to account for why some people get autoimmune diseases and others don’t. To find out, researchers have looked at identical twins, who share a single set of genes. Sure enough, a person whose identical twin has an autoimmune disease, allergy or asthma runs a greater risk than the average person of developing that same problem. Yet genes alone don’t explain all of the risk. For example, studies show that having an identical twin with asthma bumps the other twin’s risk up more than 60 percent. But this “concordance” rate is less than half in identical twins with type 1 diabetes and considerably lower in twins with lupus, multiple sclerosis or rheumatoid arthritis.
Proponents of the hygiene hypothesis argue that while genetics matter, environmental factors play a key role in most autoimmune disorders, which surged in developed nations in the late 20th century (graph, left, shows increases in some diseases from baseline levels in various European nations). The hygiene hypothesis originated when British researcher David Strachan noted in 1989 that people with lots of siblings had less hay fever than those with fewer siblings. The hypothesis holds that squeaky-clean living and few routine infections in youth can leave the immune system unchallenged, leading to poor immune-cell education and aberrant reactions down the road. Plenty of studies support the theory (SN: 1/29/05, p. 68; 9/7/02, p. 150).
Parasite regulation of immunity emerged as an offshoot of this hypothesis, but intestinal worms aren’t the only pathogens steering immune development. A study in Arizona showed that attending day care, and presumedly exposure to viruses and bacteria, might limit asthma (SN: 8/26/00, p. 134).
Immune instruction might even start before birth. Bianca Schaub of University Children’s Hospital in Munich and colleagues compared the umbilical cord blood of babies born to women who, while pregnant, had spent time on a farm with those who had spent time only in towns. Newborns whose moms were on the farm developed more of the anti-inflammatory immune cells called T-regs than the others did, tilting the farm-exposed babies away from the chronic inflammation of autoimmune diseases.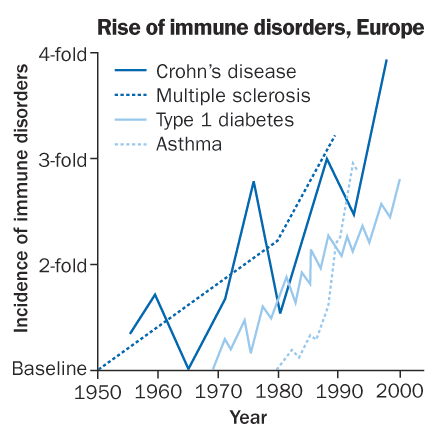 Graph: T. Dubé; Source: J.-F. Bach/NEJM 2002
Graph: T. Dubé; Source: J.-F. Bach/NEJM 2002