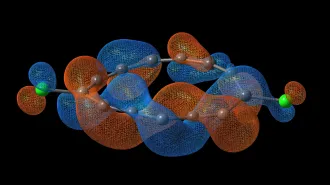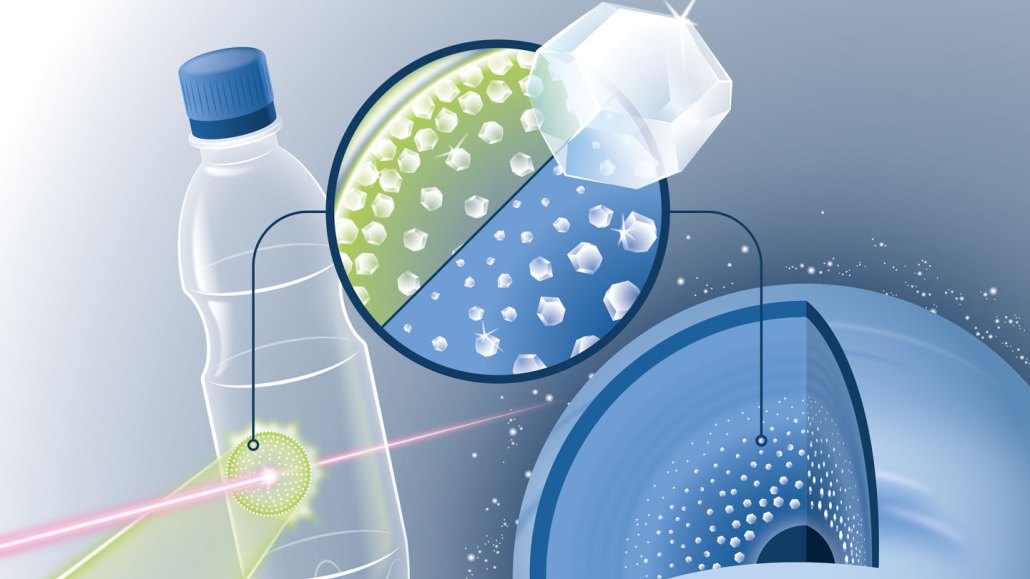
Starting from the same type of plastic found in soda bottles, scientists created tiny nanodiamonds (illustrated in inset). Researchers zapped the plastic with a laser (green) and probed it with X-rays (red). The work suggests that diamonds may form within ice giant planets such as Uranus and Neptune (lower right).
HZDR/Blaurock