Freshwater ice can melt into scallops and spikes
Water’s density quirk linked to surprising shapes
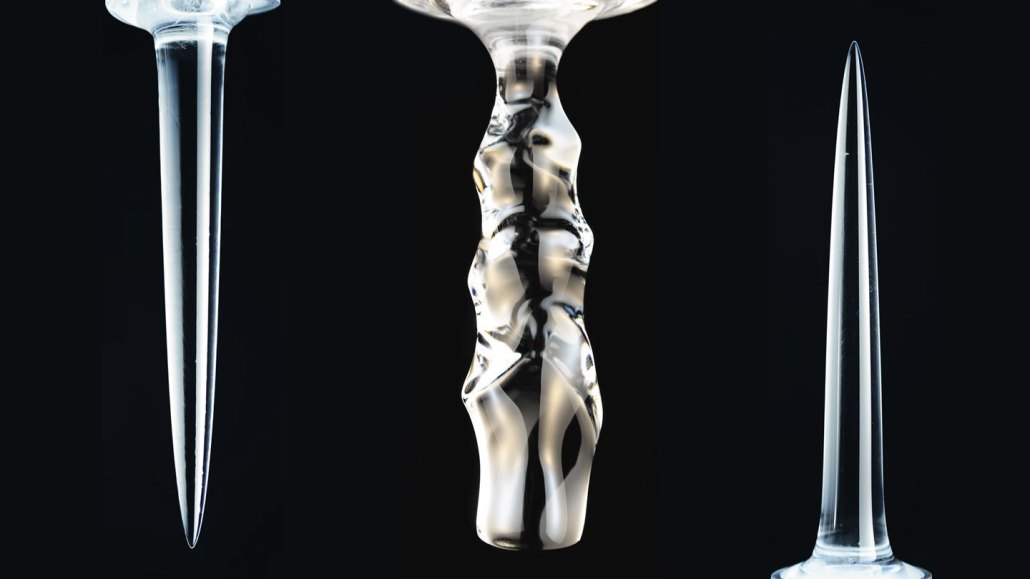
When columns of freshwater ice were submerged and melted in water, downward-facing spikes (left) formed at temperatures below about 5° Celsius, scallops (center) between about 5° C and 7° C and upward-facing pinnacles (right) above about 7° C.
Applied Math Lab/NYU







