Stowers Institute for Medical Research
A snail may hold the key to restoring vision for people with some eye diseases.
Golden apple snails (Pomacea canaliculata) are freshwater snails from South America. Alice Accorsi became familiar with the species as a graduate student in Italy. “You could literally buy them in a pet store as snails that clean the bottom of the fish tanks,” she recalls. Turns out, the snails are among the most invasive species in the world. And that got Accorsi thinking: Why are they so resilient and able to thrive in new environments?
She began studying the snails’ immune systems and has now found they are not the only parts of the animals able to bounce back from adversity. These snails can completely regrow a functional eye within months of having one amputated, Accorsi and colleagues report August 6 in Nature Communications.
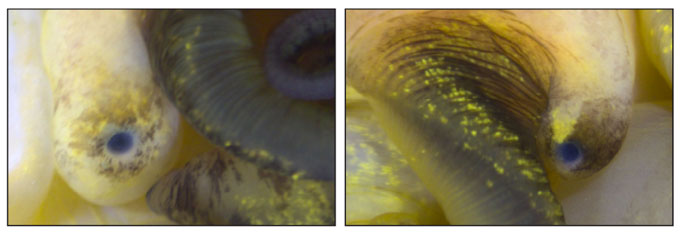
Scientists have known for centuries that some snails can regrow their heads, and research has revealed other animals can regenerate bodies, tails or limbs. But this finding is exciting because apple snails have camera-like eyes similar to those of humans. Understanding how the snails re-create or repair their eyes might lead to therapies to heal people’s eye injuries or reverse diseases such as macular degeneration.
Accorsi, now a developmental biologist at the University of California, Davis, used the molecular scissors called CRISPR/Cas9 to genetically disable certain key genes involved in eye development and established lineages of snails carrying those mutations. Those are big steps toward making snails laboratory stand-ins for studying human eye development. Developing such model organisms can take decades, but Accorsi accomplished the feat in just a few years.
That’s record time, says Alejandro Sánchez Alvarado, a developmental biologist at the Stowers Institute for Medical Research in Kansas City, Mo., in whose lab Accorsi worked out how to genetically engineer snails. “It’s not that Alice just discovered fire. It’s that she landed on the moon,” he says. Now, “she needs to build the space station from which to launch real investigation beyond the moon.”
When Accorsi snipped off a snail’s eye, the eye grew back in just under a month. But it probably took about three months for the new eye to fully integrate with the brain to restore full vision, Sánchez Alvarado says. Humans don’t regrow damaged eye parts and certainly not a whole eye. Even a transplanted eye has not yet successfully wired into a recipient’s brain.
Besides the structural similarity of human and snail eyes, both species use the same genes to form eyes. In particular, snails, like humans, need the PAX6 gene to grow eyes, Accorsi found. Snails in which she disabled that gene don’t develop eyes. Other body parts develop normally, but the eyeless snails rest on their backs in the bottom of the tank and are unable to right themselves, crawl around or find food. They didn’t move, even if the researchers flipped them over. But the snails grew to adulthood when the researchers hand-fed them leftover organic lettuce from the institute’s salad bar. That inability to forage suggests PAX6 may also be important for brain development.
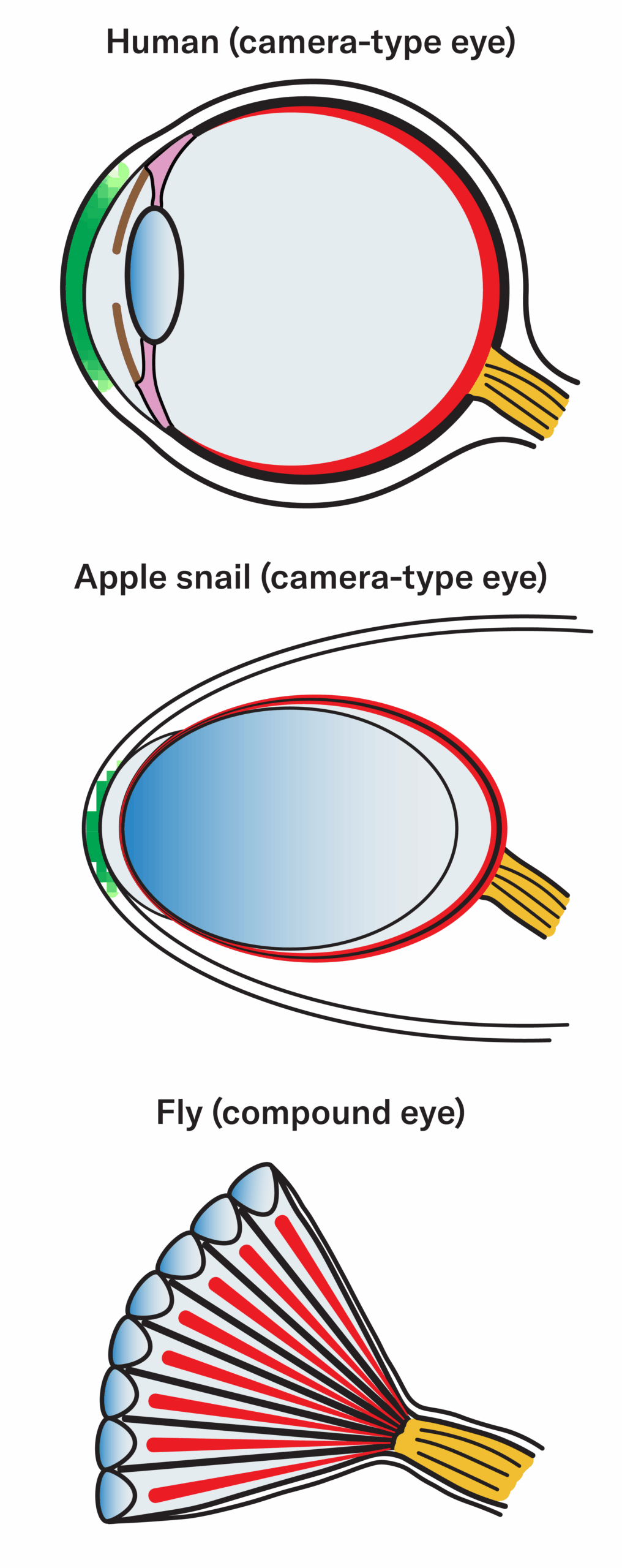
The eyes have it
Golden apple snails (middle) have camera-like eyes similar to humans (top). A camera-like eye has a single chamber, a lens, cornea and light-gathering cells in a retina at the back of the eye. The similarity could make snails a good organism for studying eye development and regeneration. Currently, much eye research is done with fruit flies (bottom), which have compound eyes. Both snails and fruit flies have many of the same genes that govern eye development in humans.
Discovering the basis of eye regeneration in snails won’t lead to immediate cures for people, says Henry Klassen, an ophthalmologist and stem cell researcher at the University of California, Irvine, who wasn’t involved in the work. But knowing that it is possible to regenerate eyes can be “like a beacon of light,” he says. “You can at least start asking questions like, ‘Where’s the hang-up? How far along the similar path do things go [in humans], and what genes, for instance, intervene or have been added to suppress [regeneration], or fail to respond?’”
Snails have other genes known to be important in human eye development. The secret to regenerating eyes is probably in the molecular switches that control when those genes are active, the researchers say. It’s possible that humans already have the same switches and researchers would just need to activate them at the right time and place to regenerate eyes, Accorsi says. Or regeneration may be controlled by a switch or switches that snails have, and humans don’t.
The researchers must first “understand what the music score of regeneration is for [the snails], and then transcribe that score,” Sánchez Alvarado says. “It’s going to be the same instruments, the same orchestra, the same genes, that allow the orchestra to play that score.” The trick is finding the right conductor.






