New type of catalyst could aid hydrogen fuel
Ferroelectric substances might offer the right combination of catch and release
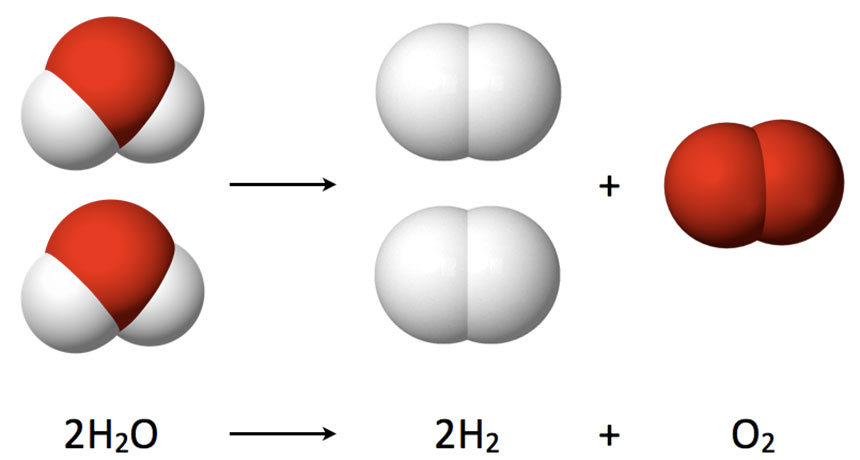
SPLIT UP Hydrogen, a clean-burning fuel, can be extracted from water molecules by splitting oxygen atoms from the hydrogens. A new computer simulation suggests a strategy for catalyzing that reaction that has the potential to be more efficient than current methods.
JSquish/Wikimedia Commons (CC BY-SA 3.0)







