Refined ‘three-parent-baby’ procedure improves chances for healthy infant
Transferring cell nuclei hours after fertilization reduces risk of passing on faulty mitochondria
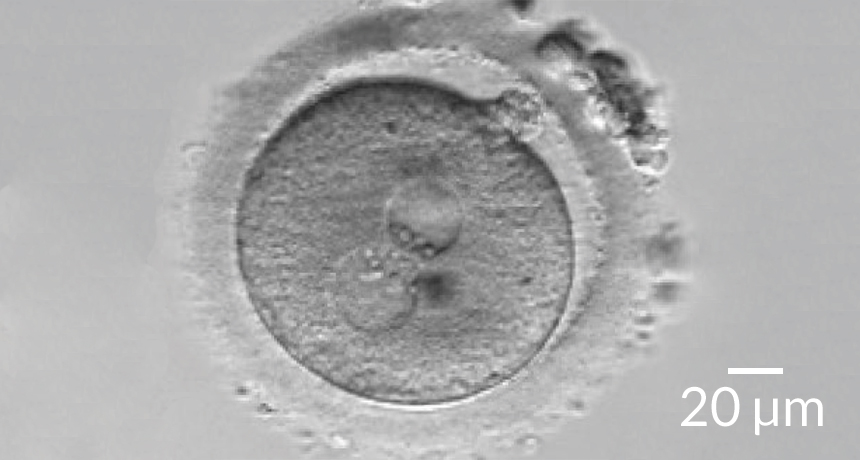
REPLACEMENT REFINED Researchers have improved a technique that transfers pronuclei (round craters, center) from an egg with diseased mitochondria to a donor egg with healthy mitochondria.
L.A. Hyslop et al/Nature 2016