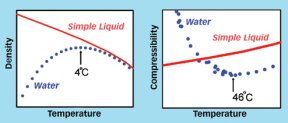
Water covers more than two thirds of our planet, makes up 60 percent of our bodies, and sustains our lives and lifestyles in countless ways. Although simple H2O may seem like the most ordinary substance in the world, it’s actually one of the strangest. Almost every other liquid contracts as it approaches its freezing point, but water expands as it freezes. If most liquids are supercooled, so that they remain liquid below their freezing temperatures, their capacity to absorb heat decreases–but water’s skyrockets. Likewise, the compressibility of most liquids doesn’t change when they’re supercooled, but water’s compressibility shoots up.
Perhaps strangest of all is that despite water’s simplicity and ubiquity, scientists have yet to unravel why it’s so peculiar.
Now, members of a small community that specializes in the microstructures of fluid and frozen liquids say they’re closing in on an understanding of the most vexing quirks of water. “A coherent interpretation of water’s properties is beginning to emerge,” chemical engineer Pablo G. Debenedetti of Princeton University and physicist H. Eugene Stanley of Boston University said in the June 2003 Physics Today.
By means of theoretical analyses, computer simulations, and laboratory experiments, scientists have traced water’s peculiar properties to variations in the microscopic arrangements of its molecules. A growing body of evidence even suggests that both everyday, familiar water and supercooled water are actually blends of two distinct liquid water forms that are never seen in their pure states.
In the course of their investigations, researchers have also uncovered unexpected parallels between water’s properties and those of silicon and other substances. These include silicon dioxide, or silica, the most abundant mineral in Earth’s mantle and crust. By exploring the newfound correspondences, scientists may better understand and predict behaviors of some of Earth’s most important materials.
Computer water
Research has been riding a wave of supercooled water since the 1970s. That’s when scientists first began to uncover the strange ways of subzero water, which is common in clouds. In particular, when investigators plotted how the heat capacity, compressibility, and several other properties change in supercooled water as the liquid’s temperature drops under ordinary pressures, the researchers noted that the extrapolated lines went off the charts at around the same temperature, –45C. That suggested something important was happening at that temperature.
Unfortunately, actually making water that cold without crystallizing it has remained just out of reach. To date, the coldest temperature at which liquid water has been observed is –42C. That’s just the kind of experimental impasse that beckons computer simulations, which can unfold in such slow motion that researchers can observe properties of a supercooled liquid before it has time to freeze.
Based on mathematical models of the interactions of water molecules, these simulations depict the various microstructures that the molecules will assume under different conditions. At the heart of these intermolecular interactions are weak bonds between a hydrogen atom on one water molecule and an oxygen atom on another. Those so-called hydrogen bonds result in transient, loose-knit clusters of molecules.
As water’s temperature decreases to and falls below the normal freezing point, more and more of its molecules self-organize into clusters that have structures based on the tetrahedron, a pyramid with a triangular base. On the molecular level, each tetrahedron consists of a central water molecule flanked by four other molecules.
In contrast, most simple liquids pack up to 12 molecules around any given central molecule to make a more compact structure that becomes even denser as it gets colder.
In principle, supercooled water’s density anomaly, as well as some of its other sub-zero zaniness, arises from its tendency to organize itself into spacious tetrahedral formations. Since tetrahedral packing is less dense than the packing of the warmer liquid, water ends taking up more space as it gets cold, even if it doesn’t freeze.
In 1992, by means of simulations, Peter H. Poole and his colleagues on a Boston University team led by Stanley identified two configurations of supercooled water molecules–the high-density form, or phase, and a low-density phase–neither of which has been observed experimentally. Both contain tetrahedral structures, but the low-density form is predicted to occupy 20 percent more volume than the high-density form.
What’s more, the simulations delineated a set of pressure and temperature values that marks the transition from the high-density to low-density phases of the supercooled water (SN: 12/5/92, p. 391). These are analogous to freezing and boiling points, where one phase of matter gives way to another.
The notion of two liquid phases of water is a radical one. In particular, “the properties of that [low-density phase] would be totally different than ordinary water’s,” says Poole, now of St. Francis Xavier University in Antigonish, Nova Scotia. “It’s a liquid of purely tetrahedral units. It would flow like molasses.”
The publication of the simulation results in 1992 prompted both excitement and skepticism. Only a few highly unusual liquids had previously been shown to have more than one fluid phase, although water has more than a dozen phases of solid crystalline ice.
Actually, the two liquid phases of water described in the simulation, may correspond to some forms of noncrystalline ice. Noncrystalline, or amorphous, ice has a disorganized molecular configuration similar to that of liquid water. Known to make up the bulk of water in comets and to coat interstellar dust grains, amorphous ice may be the most abundant type of water in the universe.
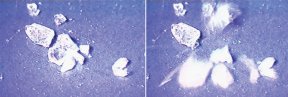
As far back as 1984, experiments by Osamu Mishima, then at the National Research Council Canada in Ottawa, and his coworkers had shown that a specific type of amorphous ice appears to alternate between two, solid phases.
After the 1992 simulations of supercooled liquid water, some scientists speculated that amorphous ice and supercooled water are essentially the same substance, and environmental conditions, such as temperature and pressure, determine whether the material is a solid or a liquid. If so, the transition that Mishima observed between the amorphous ice phases may also occur in supercooled water.
Supercooled water at some temperatures and pressures might be a mixture of the two liquid phases. Analogously, although ordinary water demonstrates a sharp transition between liquid and vapor phases that’s obvious when water boils, that crisp distinction vanishes beyond the temperature and pressure conditions known as the critical point. There, water becomes a heterogeneous mixture of liquid and vapor. That mixture has quite different properties than either of the individual phases. So, some researchers surmised that such a mixture of supercooled water’s two proposed liquid phases might explain at least some of the fluid’s bizarre features.
Lost in transition
Until recently, scientists had only seen convincing evidence of liquid-liquid transitions in liquid helium, which has unique characteristics at temperatures near absolute zero, and in extraordinary fluids–among them liquid crystals, which have molecules that tend to be highly elongated. Various experiments have investigated the hypothesis that water and other more ordinary liquids can assume more than one phase. The results have been mixed.
In 1998, for instance, Stanley and Mishima, now at the National Institute for Materials Science (NIMS) in Tsukuba, Japan, reported the first experimental evidence–albeit of an indirect sort–for a liquid-liquid phase transition in supercooled water. Theorists had proposed that if supercooled water undergoes a liquid-to-liquid phase change, then ice IV, one of the dozen or so known phases of crystalline ice, would show a discontinuity in graphs of the temperature and pressure at which it melts. Mishima and Stanley found the expected shift in ice IV’s melting conditions, but they were unable to verify that it was as sharp as predicted by the theory.
Two years later, Alan K. Soper of the Rutherford Appleton Laboratory in Chilton, England, and Maria Antonietta Ricci of the University of Rome Tre reported using neutron beams to examine the organization of molecules in supercooled water as the pressure increases. From their data and computer simulations, the scientists could infer how the water molecules packed together. They found evidence of a gradual transformation from the previously known tetrahedral microstructure for supercooled water to a more densely packed architecture.
At about that same time, a Japanese team announced the experimental observation of a liquid-liquid phase transition in phosphorus. That was the first clear-cut detection of such a transition in a simple liquid, Poole says.
More recently, a collaboration of researchers at several national and private laboratories in the United States and Canada found hints of a gradual structural change of a sort that might also occur in supercooled water. They used neutron beams and X rays to examine amorphous ices. While slowly heating high-density amorphous ice to make it transform into low-density amorphous ice, Christopher A. Tulk of Oak Ridge (Tenn.) National Laboratory and his colleagues observed not just two, but five forms of the glassy material. The team noted that many more forms may be possible.
Since then, experimental probing of the amorphous-ice transition has continued to heat up. For instance, shortly after the neutron and X-ray study, Mishima and his NIMS colleague Yoshiharu Suzuki used both naked-eye observations and laser-light scattering measurements to reexamine the transition. They again concluded that the transition occurs suddenly, like a boiling or melting point.
However, in the Nov. 1, 2003 Physical Review B (II), the U.S.–Canadian team unveils additional neutron and X-ray data that further support a gradual transition, the authors say.
To skeptics, the lack of conclusive evidence for a sharp phase transition is telling. “Much of the talk about two forms of [liquid] water is hype,” says Soper. “The confusion arises from the fact that when pressure is applied to water, its structure changes rather easily by bending hydrogen bonds,” which would not be a genuine phase change.
Distant cousins
For many researchers, the issue is no longer whether there’s a liquid-liquid phase transition of water. A sharp transition between phases is not actually needed to explain the oddities of supercooled water, says Debenedetti. It’s enough to have local variations in the packing configurations of molecules, and that has been seen in both experiments and simulations, he notes.
Scientists are now excited about phase changes in other liquids and the unexpected similarities that are turning up among materials. For instance, Poole, C. Austen Angell of the Arizona State University in Tempe, and others have been simulating the microscopic ordering of silica molecules. Angell was one of the scientists who discovered the anomalous behaviors of supercooled water.
Silica is the primary ingredient of sand, glass, and quartz. Unlikely as it seems, its molten form may be a distant cousin to water. Each has tetrahedral order and a density maximum in its liquid state. Some simulations have shown that silica undergoes a liquid-liquid transition, although the enormity of such calculations is pushing the limit of what computers can handle.
Poole suggests that the work may ultimately lead to a new perspective on silica’s phase changes and some of its other behaviors. That, in turn, could enable earth scientists to better understand how the structure of Earth’s interior came about and how it’s evolving.
Recent simulations of elemental silicon, another tetrahedrally ordered substance, also show a liquid-liquid phase transition. Angell and Srikanth Sastry of the Jawaharlal Nehru Centre for Advanced Scientific Research in Bangalore, India, describe their silicon simulations in the November 2003 Nature Materials.
Simulation studies by other scientists have indicated that silicon, like water, has a maximum density as a liquid, rather than as a solid.
“This silicon [work] is a real breakthrough,” Stanley says. “People have always thought of silicon as a perfectly straightforward element. They’re now finding that liquid silicon is anything but straightforward. . . . It’s like water.”
New experiments take supercooled liquid silicon to more than 200C below silicon’s freezing point. They show increasing tetrahedral order as the temperature decreases. This suggests that a liquid-liquid phase transition takes place at still lower temperatures, say Noël Jakse at the University of Metz in France and his colleagues in the Dec. 8, 2003 Applied Physics Letters.
Using a mathematical model, Francesco Sciortino and his colleagues at the University of Rome La Sapienza recently discovered indications that any substance with a maximum density in its liquid phase must also undergo a liquid-liquid phase transition. The researchers analyzed patterns of energy highs and lows–an “energy landscape”–associated with various molecular configurations. They described their analysis in the Oct. 10, 2003 Physical Review Letters.
The apparent similarities of liquid water, silica, and silicon are more than just coincidences, the Italian team proposes. The common properties of some very different substances may reflect patterns of structure and function that had previously eluded the scientific community.
Perhaps that’s why what began as an exploration of water’s oddities has now spilled along so many other channels.
****************
If you have a comment on this article that you would like considered for publication in Science News, send it to editors@sciencenews.org. Please include your name and location.







