Wired Viruses: New electrodes could make better batteries
With the aid of a bacteria-infecting virus, researchers have engineered cobalt oxide-and-gold nanowires that can be used as electrodes for lithium-ion batteries. The work could lead to thin and flexible power sources, the scientists say.
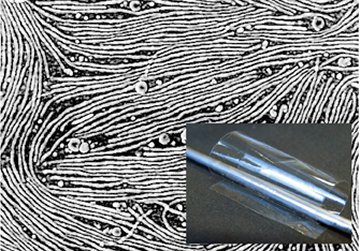
Angela M. Belcher of the Massachusetts Institute of Technology and her colleagues work with a virus called M13. It’s 880 nanometers long, 6 nm wide, and coated with a few thousand identical proteins.
Belcher’s group previously engineered the virus to serve as a scaffold to make nanoscale semiconducting wires (SN: 1/17/04, p. 46: Available to subscribers at Nanowires grow on viral templates). In the new work, the researchers used the virus to create wires of different materials suitable for electrodes in batteries.
The team added a peptide to the M13 virus’ coat protein. The peptide binds to cobalt, which, in its oxidized form, has more energy-storage capacity than does the carbon in the negative electrodes, called anodes, used in commercial batteries.
After growing millions of copies of the engineered virus, the researchers incubated them in a solution of cobalt chloride and then put the cobalt-bound virus in another solution to oxidize the metal. With this process, “you can grow these really beautiful nanowires,” says Belcher.
The team tested the wires as the anode in an experimental battery. They found that the cobalt oxide anode has about twice as great a storage capacity as a traditional carbon-based anode does.
Next, Belcher and her colleagues set out to improve the anode’s performance. They engineered the coat of the cobalt-grabbing virus to incorporate another peptide—one that binds to gold. The new hybrid anodes, made of cobalt oxide and gold, had 30 percent more storage capacity than the cobalt oxide anodes, the researchers report in an upcoming Science.
The strategy of designing a virus to attract different metals is one of the “astounding” features of this work, says Trevor Douglas, a materials chemist at Montana State University in Bozeman. “We want to be able to design complex composite materials [because] it’s unlikely that a single type of material will give you all the properties you want,” he says.
In the final part of their study, Belcher and her colleagues constructed a prototype battery by painting a layer of the virus wires onto a polymer film. In this case, the wires served as the battery’s positive electrode, or cathode, and the researchers used lithium on the other side of the film as the anode. Incorporating virus-based electrodes into flexible films, which can be 100 nm to a few micrometers thick, could lead to batteries “that take the shape of what you want to power,” says Belcher.
Her group is now working on virus-produced cathodes and plans to test other electrode and polymer materials in a prototype system.