Nitrous oxide fingered as monster ozone slayer
Most people know nitrous oxide as the laughing gas that dentists reserve for drill-phobic patients. But once it enters the atmosphere, N2O is no laughing matter. New calculations indicate that it has risen to become the leading threat to the future integrity of stratospheric ozone, Earth’s protective shield against the sun’s harmful ultraviolet rays.

Currently, Freon and other chlorofluorocarbons — or CFCs — are the leading source of ozone thinning, especially in the hole that forms annually over Antarctica. The surprise is not that N2O is also ozone-toxic. That’s been known for decades. What’s new is a measure of how its ozone-destroying potency compares to CFCs, specifically to one known as CFC-11.
Calculations by a trio of scientists from the National Oceanic and Atmospheric Administration in Boulder, Colo., now indicate that each molecule of N2O is almost one-fiftieth as effective at depleting ozone as is CFC-11.
Which may not sound like much — except it is, the NOAA scientists emphasize. Owing to its roughly 100-year survival time in the atmosphere (a lifespan comparable to CFCs) and the huge quantities released each year, N2O stands poised to become a potent player in the thinning of global stratospheric ozone. Indeed, “We found that if you look ahead, N2O will remain the largest ozone-depleting emissions for the rest of the century,” notes team leader A.R. Ravishankara.
A paper describing the new analyses was posted online today in Science.
CFCs not only are very potent agents of ozone destruction, but also have been released in huge quantities and are long-lived. The bottom line: Once these pollutants enter Earth’s upper atmosphere, they linger, catalyzing damage for decades.
As such, they deserve most of the blame for the overall five-to six-percent thinning in stratospheric ozone that has developed in the past half-century or so, the NOAA scientists say. But owing to the 1987 Montreal Protocol, a United Nations treaty that has restricted or banned use of the most ozone-toxic chemicals, stratospheric ozone thinning has peaked and now appears to be falling.
NOAA calculations now suggest that gains made under the Montreal Protocol will slow or halt, owing to the huge and rising contributions of a pollutant that also imperils ozone — but remains ignored by the powerful treaty. Owing to a twist of fate, the treaty’s success in limiting CFC emissions will also begin intensifying N2O’s potency.
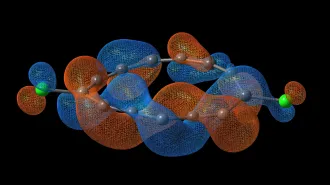
To understand why, Ravishankara says, it helps to know how CFCs and N2O damage ozone. Solar ultraviolet radiation breaks CFC molecules apart, creating chlorine and chlorine oxides. “These are what destroy ozone,” he says, not the parent CFCs. Similarly, N2O doesn’t directly damage ozone. Chemical reactions in the stratosphere must first strip away one of that molecule’s nitrogen atoms — forming nitric oxide, or NO. This stripped down molecule, he explains, is what actually wreaks havoc with ozone.
“Nitrogen oxides and chlorine oxides kind of oppose each other in destroying stratospheric ozone,” the scientist explains. “In other words, N2O offsets the ability of chlorine oxides to destroy ozone. And vice versa.” In the new paper, he says, “We have calculated the ozone-depleting potential of N2O to be roughly 50 percent larger when chlorine levels return to the year-1960 level.”
As N2O pollution goes, dentists are very bit players. Deforestation, animal wastes and bacterial decomposition of plant material in soils and streams emit up to two-thirds of atmospheric N2O.
However, emissions from such natural sources appear fairly static, Ravishankara said at a briefing yesterday. That’s in stark contrast to N2O releases from processes fostered by human activity, such as the nitrogen fertilization of agricultural soils and fossil-fuel combustion. These anthropogenic emissions of the pollutant have been growing steadily, he says, to where they now boost atmospheric concentrations of N2O by roughly one percent every four years.
What all this means, the NOAA scientists say, is that N2O is now a bigger threat to future stratospheric ozone destruction than are CFCs. And if N2O emissions don’t diminish substantially, Ravishankara says, within a century they could eventually slay 40 percent as much stratospheric ozone each year as CFCs did at their peak.
Reporters asked the NOAA team what can and should be done, but the scientists simply argued that finding answers was the responsibility of policymakers. However, Ravishankara observed that because most N2O releases are so diffuse, limiting them will prove much more challenging than simply mandating controls on tailpipes or smokestacks.
Yet success would yield a doubly whammy, notes coauthor John Daniel. The reason: N2O is also a greenhouse gas.