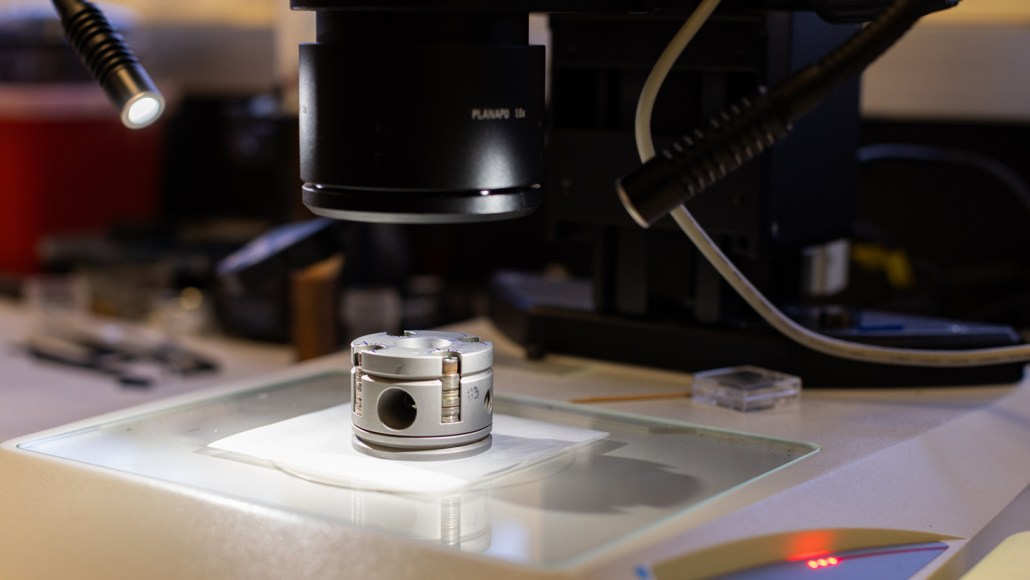
A diamond anvil cell (shown) is used to increase the pressure, and then rapidly release it, on a new record-breaking superconductor.
Anthony Gollab/University of Houston
Nothing says “the ’90s are back” like a challenge to a high-temperature superconductor record set in 1993.
By squeezing a material to high pressure and then rapidly releasing it, scientists reduced the amount of cooling it needs to become a superconductor. The mercury- and copper-based compound remained a superconductor up to temperatures as high as 151 kelvins (–122.15° Celsius) under atmospheric pressure, researchers report March 9 in Proceedings of the National Academy of Sciences. If the result holds up, it would be the highest-temperature superconductor known to exist at atmospheric pressure, by about 18 degrees.
Electricity courses through superconductors without resistance, a property that could lend itself to a variety of technical applications, from powerful electromagnets to power transmission. But all known superconductors must be chilled well below room temperature to function, which limits their applicability. (Earlier claims of room-temperature superconductivity didn’t hold up.)
Higher temperatures are possible when materials are squeezed to extreme pressures, but that makes the materials difficult to study and to use. For example, a compound of lanthanum and hydrogen is superconducting up to 260 kelvins (–13.15° C), at a pressure almost 2 million times that of Earth’s atmosphere. That’s the highest superconducting temperature ever confirmed.
The new result could put phenomena that were previously difficult to access within easier reach, says physicist James Hamlin of the University of Florida in Gainesville, who was not involved with the work. “There’s so much interesting stuff that happens at high pressure, and the idea that we might be able to bring some of that back to ambient pressure is really exciting.”
The new superconductor is part of a class of copper-oxide compounds called cuprates. In the 1980s and 1990s, cuprates repeatedly broke superconducting temperature records. But eventually, progress stalled. In 1993, a mercury-based cuprate called Hg-1223 reached 133 kelvins at atmospheric pressure. That record has stood ever since. Now, like Trapper Keepers, acid-wash jeans and scrunchies, cuprates are back.
The researchers increased the pressure on samples of Hg-1223 to between 10 and 30 gigapascals (about 100,000 to 300,000 times atmospheric pressure) by squeezing the material between two diamonds, in a device called a diamond anvil cell. That pressure raised the temperature at which the resistance of the material began to drop.
Then the scientists set the temperature very low, around 4 kelvins, and suddenly released the pressure. That may have helped prevent the material from reverting back to its original state: When heated back up, the material retained signs of higher-temperature superconductivity, but at atmospheric pressure.
The technique is no easy feat, says physicist Paul Chu of the University of Houston, which explains why the previous record has stood since the ’90s. “When you withdraw the pressure at such a high speed, everything flies apart,” Chu says. The diamonds can break or electrical contacts can be severed, ruining the measurement. But when Chu and colleagues’ experiments worked, the material transitioned to a superconductor at temperatures close to 150 kelvins.
Manipulating a material by rapidly changing the conditions is known as quenching. It can lock in desirable properties of a material before they have time to change. Temperature quenching, for example, is used in the manufacture of steel — think blacksmiths putting hot metal into a bath of water to rapidly cool it.
“It’s an exciting method,” says theoretical chemist Eva Zurek of the University at Buffalo in New York. And it could be applied to other superconducting materials. “But it won’t work in all cases.” The superconducting materials have to be metastable, meaning they can retain their properties, at least temporarily, when the pressure is removed.
Chu and colleagues reported that the material retained its higher-temperature superconductivity for at least three days when stored in liquid nitrogen (a temperature of 77 kelvins), but keeping it in warmer conditions (around 200 kelvins) made the material’s properties deteriorate.
In the experiments, the evidence for superconductivity was that the resistance dropped dramatically when the material was cooled below a certain temperature. But the researchers did not explicitly show that the resistance went to zero — a more stringent test of whether a material is a superconductor. Such measurements can be difficult in diamond anvil cell experiments.
However, this material is already well-known to be superconducting. Compared with controversial, discredited research that has plagued the field of superconductivity in recent years, “it’s a much more straightforward claim,” Hamlin says. “It’s not coming out of left field in any sense.”