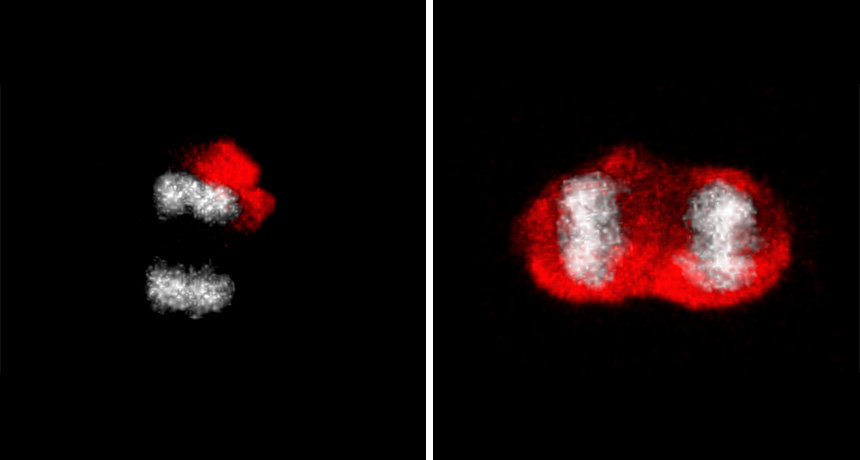
THE GREAT DIVIDE Young brain stem cells (one shown dividing, left) pack old proteins tagged with ubiquitin (red) into one daughter cell. Old cells (one shown dividing, right) lose a barrier that segregates the damaged proteins. DNA is shown in gray.
D. Moore et al/Science 2015
Breaking down barriers usually sounds like a good thing, but not for aging stem cells.
When young brain stem cells split in two, they can wall off damaged proteins in one daughter cell, leaving the other spry and ready to divide again, researchers report in the Sept. 18 Science. With age, the barrier sequestering the damaged proteins breaks down, spilling cellular garbage into both cells, the team also discovered. The spillover may diminish older stem cells’ ability to divide and replenish tissues. Learning why such barriers fall apart may eventually lead to new kinds of antiaging therapies.
Researchers have previously demonstrated that yeast, fruit fly cells and some types of human cells grown in lab dishes divvy up proteins unequally. The new study is the first to show that lopsided segregation happens in the brain, says developmental biologist Eddy De Robertis of UCLA. He was not involved in the study, but his lab has shown that cells unequally divide proteins studded with a molecule called ubiquitin.
Ubiquitin clinging to a protein is one signal that the protein is ready for the garbage or recycling bin. In the new study, researchers tracked ubiquitin-studded proteins as mouse neural stem cells divided into two cells: one remaining a stem cell and the other becoming a neuron, or nerve cell.
“We didn’t know who would stay clean, the stem cell or the daughter cell,” says study coauthor Sebastian Jessberger, a neuroscientist at the University of Zurich. “It could be that the stem cell keeps the dirt.”
But that’s not what the researchers found. Instead, “the stem cell remains pristine and the daughter cell takes with it whatever baggage there is,” says De Robertis.
As cells age, though, old proteins can be found in both newly divided cells, the researchers discovered, indicating that the barrier that kept the “dirty” proteins in daughter cells has crumbled. Brain stem cells from middle-aged mice (9 months old) no longer divided as often as ones from 1.5-month-old mice did, the team found. That finding suggests that inheriting old junk can make normally vigorous stem cells decrepit.
Jessberger and colleagues aren’t yet sure how the barrier works. “There’s not a complete fence between one side of the cell and the other,” he says. Instead, he envisions a mesh of proteins inside the endoplasmic reticulum — a network of tubes in which cells make and package proteins — that catches proteins and other molecules related to aging, while letting others slip through.
A protein called lamin A may be part of the mesh. Children who have a mutation in the gene encoding lamin A develop a fatal premature aging syndrome called Hutchinson-Gilford progeria. The mutation leads to a buildup of a form of the lamin A protein known as progerin, which also builds up in the cells of aging people who don’t carry the mutation. Progerin interferes with lamin A’s ability to build a scaffold that supports the nucleus, the compartment where cells store DNA.
Forcing young mouse brain stem cells to make progerin also weakened the barrier that excludes aging proteins from the stem cells, the researchers discovered. The protein probably isn’t the only component of the barrier, but the researchers don’t yet know which other proteins are involved or how the barrier sorts aging factors.
“Finding that barrier’s molecular nature will be very interesting,” De Robertis says.
Inheriting cellular garbage might not be so great for newborn daughter cells, and may help explain why some brain cells die soon after they are born, Jessberger says. On the other hand, many brain cells live a long time, leaving plenty of time to clean up the mess handed down by their mother stem cells. And the new neurons may get helpful heirlooms from their mother cells, Jessberger says. “It doesn’t mean it’s always bad.”






