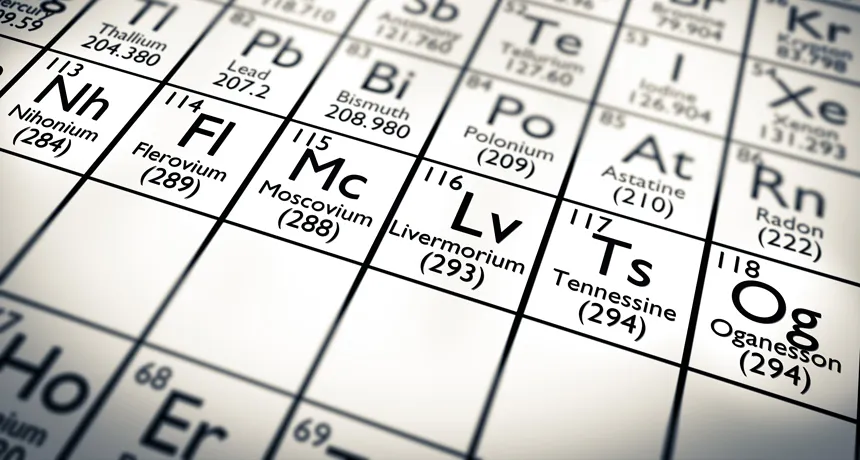
NAME GAME First created in a lab in 2002, element 118 was officially dubbed oganesson in 2016. New studies predict some of the element’s odd properties.
Antoine2K/Shutterstock
The first 117 elements on the periodic table were relatively normal. Then along came element 118.
Oganesson, named for Russian physicist Yuri Oganessian (SN: 1/21/17, p.