The Bad Seed
Rare stem cells appear to drive cancers
While some brain tumors are treatable, many are a speedy death sentence despite the best efforts of physicians. A neurosurgeon may carefully cut out every sign of a brain tumor, but new cancer cells quickly arise to take the original tumor’s place. The cancer may even overcome toxic chemicals and intense beams of radiation, powerful weapons that kill rapidly dividing cells and suppress the growth of many tumors.
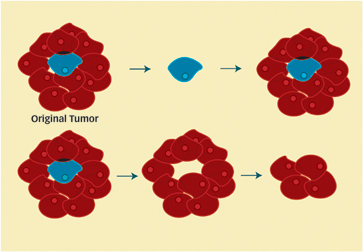
Why are many brain tumors and other cancers so difficult to treat? The answer may lie in the cancer stem cell, an old idea that has been given new life in recent years.
For decades, cancer researchers have wrestled with two competing visions of tumors. In one scenario, all the cells of a tumor are pretty much the same. That is, they have an equal capacity to divide and form new tumors.
In the other scenario, only a few select cells from a tumor have the capacity to initiate new, full-fledged tumors. These bad seeds are the cancer stem cells.
Oddly enough, it took the study of leukemia, a blood cancer in which there typically is no actual tumor, to initially prove the existence of cancer stem cells. A decade ago, John E. Dick of the University of Toronto led a research team that harvested cancer cells from people with leukemia and showed that only some of these blood cells could reproduce the disease in rodents.
Until recently, scientists weren’t sure whether stem cells also play a role in malignancies producing solid tumors. Early last year, however, Michael F. Clarke of the University of Michigan Medical School in Ann Arbor and his colleagues reported that a small minority of human breast cancer cells, perhaps just 1 in 100, forms tumors when implanted into mice. Later in 2003, two research teams independently presented evidence that cancer stem cells underlie brain tumors as well.
“I think the cancer–stem-cell hypothesis will apply to every kind of cancer,” says Dick.
Researchers are now racing to identify tumor-forming stem cells in skin, lung, pancreatic, ovarian, prostate, and many other cancers. “We simply need to know what the cells are that give rise to the tumor. It’s an unknown for virtually all tumor types,” says Tyler Jacks, director of the Center for Cancer Research at the Massachusetts Institute of Technology (MIT).
Biologists are also exploring how cancer stem cells originate. Researchers suspect that the dangerous cells may arise from mutations in the normal stem cells that sustain various tissues. This type of inquiry is so hot that Stanford University has just created an institute that brings together cancer researchers and stem cell biologists in an unprecedented research marriage.
The concept of cancer stem cells could change notions of how cancers spread and how tumors should be treated. For example, some current cancer drugs may turn out to kill most cells in a tumor but to spare the stem cells, thereby setting the stage for a relapse. “If we don’t eliminate those cells, then they will just re-form tumors,” says Clarke.
One in a million
Normal stem cells in healthy organs have two defining characteristics. First, they show a kind of immortality that scientists call self-renewal because these cells can divide indefinitely to produce more copies of themselves. Second, a stem cell is unspecialized, but it can produce progeny that mature into the various cell types of, say, the brain or the immune system. Once this maturation occurs, the stem cell heirs may divide rapidly but only a limited number of times.
The blood system has the best-described normal stem cells. In the 1980s, Dick and other researchers characterized the mouse stem cell that resides in bone marrow. This hematopoietic stem cell can give rise to both red blood cells and the various white blood cells of the immune system. In fact, a single mouse hematopoietic stem cell can reconstitute an animal’s entire blood supply.
After finding the mouse stem cell, Dick and his colleagues began looking for a human counterpart that could form human blood cells when transplanted into immune-deficient mice. As they struggled with that challenge, they wondered whether human leukemia cells would reproduce in immune-deficient mice. The researchers reported in 1997 that some of the human cancer cells that they harvested from patients would grow in the lab animals.
“We showed that roughly one cell in a million, when transplanted into a mouse, had the capacity to regrow the disease,” says Dick.
Those rare cells, the researchers concluded, could be considered cancer stem cells. The team then characterized those dangerous cells by distinctive proteins on their surfaces. It turned out that the leukemia stem cells sport a protein, called CD34, that healthy hematopoietic stem cells also carry but other cells don’t. Furthermore, the human-leukemia stem cells consistently lack another protein, CD38, that most other leukemia cells have.
Since then, scientists have unearthed stem cells in other blood cancers, most recently multiple myeloma. In this disease, antibody-making plasma cells accumulate in and destroy bone marrow.
However, the plasma cells don’t divide very often, notes William Matsui of Johns Hopkins Medical Institutions in Baltimore. So, he and other investigators have long suspected that some other cell generates the multitude of cancerous plasma cells.
In the March 15 Blood, Matsui and his colleagues present evidence that the stem cells in multiple myeloma are a subset of immune cells known as B cells. The stem cells both self-renew and develop into the mature plasma cells that mark multiple myeloma, the researchers found.
That finding makes sense because earlier work showed that B cells give rise to plasma cells during normal blood development, says Matsui.
Wake-up call
The concept of cancer stem cells has been around since at least the 1950s. “The hypothesis was right, but [scientists] couldn’t come up with the experiments needed to prove it,” says Clarke.
Aware of Dick’s work on leukemia stem cells, Clarke in the 1990s became attracted to the idea that solid tumors also have stem cells. One day, when examining cells from a testicular cancer, he noticed that a few of the cells bore a surface protein common to immature fetal cells. “That made me think, ‘Holy cow, this is a stem cell disease,'” recalls Clarke.
The biologist decided to look for stem cells in a more common disease, breast cancer. In a strategy developed by Clarke’s colleague Muhammad Al-Hajj, the researchers initially sorted human breast cancer cells into populations defined by their surface molecules. The scientists then transplanted the various cell populations into the mammary tissue of immune-deficient mice to see whether they would grow tumors.
The team eventually homed in on a group of cells bearing a protein called CD44 but lacking other surface proteins common to breast cells. Whereas it takes injections of many thousands of unsorted breast cancer cells to trigger a tumor in such rodents, transplanting as few as 100 of the CD44-bearing cancer cells reliably generated the cancer. The scientists could even isolate the same subset of cells from the new tumor and transplant them into another mouse to invariably generate another tumor.
This study “caused people to wake up,” says Dick. The scientific community found the work, appearing in the April 1, 2003 Proceedings of the National Academy of Sciences, to be compelling evidence that a rare subset of cancer cells creates tumors in the breast, he notes. It also prompted investigators to consider whether other solid tumors harbor stem cells.
Not long after Clarke’s paper came out, two research groups working independently reported that cells with stem cell characteristics were present in children’s brain tumors. One team was headed by Peter B. Dirks of the Hospital for Sick Children in Toronto; the other, by Harley I. Kornblum of the University of California, Los Angeles.
Dick’s work with leukemia stem cells had inspired Dirks to think differently about solid brain tumors. “A lot of cancer research involves studying the whole tumor mass,” explains Dirks.
“People tend to grind up the solid mass and not consider each individual cell in the tumor.”
Taking a different approach, he and his colleagues separated brain tumor samples into individual cells. Next, the team looked among those cells for a surface molecule that had been recently identified on stem cells in a healthy human brain.
Dirks’ team hypothesized that this molecule, CD133, might also mark brain tumor stem cells. Indeed, small numbers of cells displaying CD133 reside in a variety of brain tumors, the researchers found.
In a laboratory dish, the cells reproduced and also differentiated into the same varieties of brain cells seen in the original tumors, the researchers reported in the Sept. 15, 2003 Cancer Research. In contrast, the growth of other tumor cells isolated petered out.
In the Dec. 9, 2003 Proceedings of the National Academy of Sciences, Kornblum and his colleagues published their own report that CD133-bearing cells isolated from pediatric brain tumors behave as stem cells. The two studies’ results are “remarkably similar,” Kornblum says.
His group also described transplanting the newly identified cells into the brains of newborn rats. The cells migrated throughout the brain, produced nerve cells and other kinds of brain cells, and continued to proliferate for up to a month.
Kornblum’s group is now conducting longer studies to see whether brain tumors will arise in the animals receiving the transplants. Dirks and his colleagues have also begun transplanting cells into the brains of rodents for a similar test.
A dangerous cell
One of the main issues regarding cancer stem cells is whether they’re normal stem cells gone awry or differentiated cells that have acquired stem cell characteristics. The former scenario appeals to most scientists, although they acknowledge it’s largely unproved.
Because it can replicate endlessly, a normal stem cell is a “very dangerous cell” that’s poised on the edge of becoming cancerous, says Dick. The potentially endless reproduction of a stem cell also allows enough time for cancer-promoting mutations to accumulate in such a cell, he explains.
The cancer–stem-cell hypothesis could explain why many cancers are resistant to radiation and drugs. Normal stem cells are unusually hardy and possess molecular pumps similar to the ones that some cancer cells use to flush out chemotherapy agents, notes Kornblum.
The discovery of cancer stem cells is forcing scientists to reconsider how they look for tumor-fighting drugs. “Everyone has been concentrating on proliferation,” says Clark. Traditionally, researchers screen for compounds that kill dividing tumor cells, but stem cells are often quiescent, only occasionally spawning progeny that then rapidly proliferate.
“The biology of the tumor you see may not be the same as the biology of the stem cell. You’re never going to cure someone unless you hit the stem cell,” says Matsui.
Scientists battling leukemia, the disease in which a cancer stem cell was first isolated, have been focusing on this new target for a few years, says Dick. As one example, he points to a 2002 study in which Craig T. Jordan of the University of Kentucky Medical Center in Lexington and his colleagues identified compounds that specifically kill leukemia stem cells derived from patients.
The research on cancer stem cells also threatens to upend thinking on how cancers spread, or metastasize. Conventional theories hold that metastasis is an evolutionary process in which a small number of cells within a primary tumor gradually accumulate the genetic mutations that enable them to spread to other tissues and establish new tumors. An alternative model now being put forth is that many cells in a primary tumor spread in the body, but a second tumor arises only when a rare stem cell reaches a new site.
Scientists have proposed that identifying cancer stem cells from various types of tumors will help them isolate the long-sought normal stem cells in tissues such as the prostate gland and the breast. “Tracing back from the tumor to that cell population will allow us to identify these critical cells in normal tissue,” says Jacks, who is a Howard Hughes Medical Institute investigator at MIT.
“It’s a new field and there’s a ton of work that needs to be done,” concludes Clarke.