Better by Design
Avoiding undesirable traits from the start could help chemists make molecules less meddlesome
Chemistry is all about sparks — bonds break, beakers bubble, reactions rule. But a growing number of researchers are obsessed with chemistry’s quieter side. Rather than vigor and vim, they seek a calm predictability.

These scientists are calling for a new approach to chemical design: They want compounds that do one job well — whether rubber that bounces back or nail polish that shines — but the action needs to end there. No multitasking, thank you very much.
Many of today’s chemicals — in packaging, cleaning products, furniture and elsewhere — go where they should not go and do more than they were designed to do. Bisphenol A, a common ingredient in polycarbonate plastics, has made headlines for getting into the body and interfering with tissue development and function (SN: 7/18/09, p. 5). Flame retardants new and old persist in the environment, contaminating soil, waterways and wildlife (SN: 4/24/10, p. 12). And a new analysis, reported online January 14 in Environmental Health Perspectives, finds that the blood and urine of 99 percent of pregnant American women tested contain a laundry list of chemical interlopers, including various PCBs, pesticides, PFCs, PBDEs, phthalates and the rocket-fuel ingredient perchlorate.
Unless there is a fundamental shift in the way that chemicals are created from the outset, the next generation of compounds will probably be just as meddlesome, says Michael Wilson, associate director of the green chemistry center at the University of California, Berkeley. Currently more than 30 million metric tons of chemicals are produced in or imported to the United States each day, a quantity that would fill a line of tanker trucks 10,000 miles long. And industrial chemical production is expected to double in the next quarter century, outpacing population growth.
Ensuring that tomorrow’s molecules are more mild-mannered than today’s is doable, Adelina Voutchkova of Yale University’s green chemistry center and colleagues argued last year in Chemical Reviews. (One of the paper’s coauthors is Paul Anastas, known widely as the father of green chemistry.) Chemists have long designed compounds to have specific properties, Voutchkova says. The same chemists should be able to design compounds that lack unwanted properties.
An analysis published in Tetrahedron last year suggests that it is possible to identify the traits that help chemicals get through cell membranes and gum up cellular machinery. Using existing data on the personalities of more than 500 known toxic chemicals and an additional 130,000-plus commercial chemicals, Voutchkova’s team came up with a set of parameters for predicting qualities that new compounds would ideally lack.
Some of the toxic compounds, for example, had atoms in tight three- or four-member rings, an awkward conformation that can make the chemicals highly reactive and more likely to inflict cellular damage than those with larger rings.
Of course, lacking small rings doesn’t ensure harmlessness. Like setting up a friend on a blind date, fulfilling negative requirements such as “no musicians” or “not an urbanite” doesn’t necessarily make for good chemistry. But the assessment suggests that when traits are taken together, there is a relatively well-defined plain-Jane “chemical space” where compounds that tend to behave themselves can be found.
“We are trying to give chemists a tool that says, ‘OK, rationally, when I look at this new molecule that I haven’t seen before, can I assess how likely is it to be absorbed by the body?’ ” says Voutchkova. “We hope in five to 10 years that companies will have some rational strategies for finding safe alternatives as opposed to finding them by chance.”
Do not enter
Little is left to chance in the pharmaceutical industry, in which chemists are adept at creating molecules to push biological buttons. A metric known as “Lipinski’s rule of five” is commonly used in drug design to figure out if a compound taken orally is biologically active and therefore a good drug candidate.
“We’re basically turning that on its head,” says Julie Zimmerman, also of Yale and a coauthor of the paper in Tetrahedron. The physical and chemical properties that give molecules access to people’s cells can be flipped to prevent access, or to minimize activity if a compound ends up where it shouldn’t.
Simple traits such as molecular weight and solubility strongly influence whether a compound will even get into the body — the way height and weight constraints keep adults off of kiddie roller coasters. Inhaled particles larger than 1 micro-meter across are less likely to be absorbed into the blood via the lungs. Particles reaching about 5 micrometers or greater will most likely be absorbed by the mucous layer or caught in the throat passage and then swallowed. For particles that make it into the GI tract, having a lot of positive or negative electrical charge can influence absorption.
And shape alone can reveal a lot about how harmful a molecule will be if it does make it into the body, Zimmerman says. If a new compound has a molecular arm that can swat an estrogen receptor, for example, it may meddle with hormonal signaling. The strength of the carbon-hydrogen bonds can help predict whether a compound will interact with enzymes that often kick off the first round of metabolism in humans.
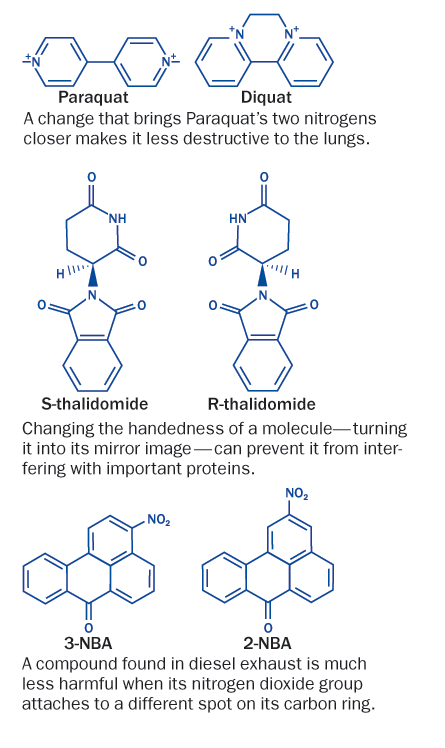
A growing body of data illustrates how tweaking a delinquent compound’s shape can rehabilitate it, Voutchkova says. A widely used herbicide known as Paraquat was found to cause serious damage when it accumulated in the lungs. Lung cells are particularly good at transporting certain nitrogen-containing compounds across membranes. A slight tweak brought the chemical’s two nitrogens closer together and the altered version, called Diquat, is much less attractive to lung cells.
Switching where the atoms in a chemical link up may also alter behavior. Two molecules that are found in airborne particulates look identical except for which carbon a nitrogen bonds to. Yet the molecules differ vastly in their ability to sabotage DNA, an international team of researchers reported last July in Chemical Research in Toxicology.
Made to fade
Whether or not a compound will break down easily and safely in the environment can also be discerned from its molecular structure, says Robert Boethling of the U.S. Environmental Protection Agency’s Office of Pollution Prevention and Toxics. Boethling has been reviewing musk compounds as part of a larger effort to identify molecular traits that influence how well a compound biodegrades.
Valued by the perfume industry for its penetrating odor, real musk comes from a sac beneath the abdominal skin of the male musk deer and has an oxygen that helps get the breakdown process going. But cheaper synthetic versions have come into widespread use. Members of one class, the nitro-musks, are ringed structures dotted with nitrogen groups.
“A person wishing to design non-persistent chemicals would have looked at that from the very beginning, from the pencil-and-paper stage, and said, forget it,” Boethling says. “It’s not going to degrade.”
Zimmerman, Voutchkova and others hope that if chemists are made aware of traits such as toxicity and biodegradability, such unfavorable compounds won’t make it past the drawing board. “Chemists are actually pretty good at designing for properties — making things that are red, for example, or taste a certain way,” says Zimmerman.
Designing a compound with features that make it friendly to the body and the environment needs to become the status quo, Voutchkova says.
Scientists outside of chemistry are eager to help. In a letter published March 4 in Science, eight scientific societies urge the EPA and the Food and Drug Administration to call on society members in other fields for expertise when assessing chemical risk and setting policy.
“Chemists are never trained to even think about what the consequences of a molecule might be,” Voutchkova says. “We aren’t trained to understand what the connection between structure, properties and biological effects might be — that’s alarming to me.”