Code Breakers
Scientists are altering bacteria in a most fundamental way
Something extraordinarily unnatural is about to take shape in Peter G. Schultz’s laboratory.

Except for its sweeping view of the Pacific Ocean, the lab itself, at the Scripps Research Institute in La Jolla, Calif., seems unremarkable. It’s a perfectly ordinary, if rather large, research facility. The researchers there work with the most commonplace of all laboratory bacteria—the ubiquitous Escherichia coli.
But this familiar bacterium is about to become unique among creatures on Earth.
Schultz and his colleagues, as well as a group at the University of Texas at Austin led by Andrew Ellington, are engineering E. coli with an altered code for translating DNA information into protein. The researchers are trying to move beyond the humdrum set of 20 amino acids that make up the building blocks for life today. They plan to add protein-building materials not found naturally in any living organism. The result will be a preternatural E. coli—an Uncoli, as the Texas group has dubbed its organism.
The first bacterium with a laboratory-induced variation in its genetic code was created in 1983. Until recently, though, scientists have focused on altering individual proteins in a test tube to learn more about the way the molecules work. Now, researchers at Scripps and Texas hope to discover how to weave new patterns into the fabric of life by creating bacteria that follow exceptional translation programs. They say that they are close to having organisms that unequivocally work with a new plan.
“I think what they’re doing is very exciting,” says bioethicist Arthur L. Caplan of the Center for Bioethics at the University of Pennsylvania in Philadelphia. “On curiosity grounds alone, it’s fascinating.”
A different life
For decades, scientists have been speculating about how life would be different if it followed an alternative genetic code. “It’s by imagining what the world would be like if it were different that will allow us to see why it is the way it is,” says evolutionary biologist Olivia P. Judson of Imperial College at Silwood Park in England.
David R. Liu, a chemist at Harvard University who had a hand in engineering the novel organism at Scripps, says, “Everybody who’s considered this problem is tantalized by the question: Is life better with a different set of amino acids?”
That had been a purely hypothetical, grass-is-greener query, but the Uncoli and its ilk could change all that. “This will be the first time we will have a nontheoretical answer to that question,” says bio-organic chemist Thomas J. Magliery of the University of California, Berkeley and Scripps.
Although the research is intrinsically intriguing, there are also practical reasons for building the novel bacteria. The unusual creatures might help clean up toxic wastes that would kill natural organisms. With the right protein components, Uncoli might spin polyester or other synthetic polymers.
Unanticipated advances may stem from this research if it provides answers to some of the most basic questions about life on Earth—and, perhaps, elsewhere in the universe.
If life on other planets uses building blocks that are very different from our own, scientists may have a hard time recognizing E.T. if they finally meet him. “We don’t really know how to detect life with a very different biochemistry,” says Michael H. New, an astrobiologist at NASA’s Ames Research Center in Moffet Field, Calif.
There are lots of stars and planets in the universe, New says, but scientists don’t know which ones might harbor life. It’s easy to guess that Earthlike conditions are necessary for developing a biochemistry like ours, he says, but where do you look for organisms made of different stuff? New speculates that the Uncoli may tell scientists something about where and how to look for life that may have an unexpected style.
“It’s kind of like a truffle hunt,” says New. “If you didn’t know that truffles like oak forests, you might spend a lot of time looking for them in pine forests and never find a truffle.” He says he hopes organisms with a different genetic code can help astrobiologists distinguish between the planetary equivalents of oak and pine forests to root out alien species.
Expanding the genetic code
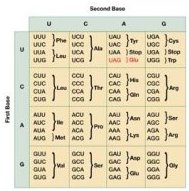
Magliery and his colleagues at Scripps say that expanding the genetic code may illuminate the evolutionary path by which life arrived at the universal genetic code we have today. The four bases that serve as letters of the genetic alphabet are arranged in 64 three-letter words called codons. Each codon stands for one of the 20 amino acids that make up proteins or for one of three stop codons that signal the end of a protein chain.
One of the first things that people notice about the genetic code is its redundancy, Judson says. Just as some languages have many words that mean snow, the genetic code has many codons that name the same amino acid. There are six different ways to say arginine, leucine, and serine, and most amino acids correspond to at least two codons. Only methionine and tryptophan are each spelled out by a single three-letter word.
Theoretically, the 64 codons could designate 64 different amino acids, but that arrangement would leave no room for error, says Judson. Any change in the DNA would alter a protein’s amino acids. Still, scientists have not settled on an explanation for why the code contains 20 amino acids as opposed to 30 or more. “We don’t even know what having 20 amino acids gets you that 16 doesn’t,” says Judson.
Some researchers suggest they may have discovered the reason behind the familiar genetic code. “Three billion years ago, it was all cutthroat competition for things that could resist errors the best,” says evolutionary biologist Stephen J. Freeland of Princeton University.
He argues that the universal genetic code mounts a championship error defense. In computer simulations, he assessed the severity of errors introduced by mutations in a million randomly generated genetic codes, and he found that the natural code “outperforms almost all of them.”
Judson points out, however, that nature itself has been experimenting with the universal code. Just take a look at mitochondria, the organelle inside cells that serve as power plants to generate energy. There, the genetic code is by no means universal. Only mitochondria in plant cells follow the rules of the standard code, she says. Mitochondria in most other organisms have pared down the code and appear to be moving toward one with only two codons assigned to each amino acid, she says. That would leave plenty of codons available to signify new amino acids.
Many organisms are already living an alternative lifestyle when it comes to the genetic code, Freeland says. Even human biology bends the rules. Human cells have added an amino acid called selenocystine to their repertoire of building blocks. In a handful of proteins, they incorporate this 21st amino acid in response to some of the codons for cystine. These proteins can’t function without selenocystine.
Selenocystine provides the perfect example of how evolution continues to shape the genetic code, says biochemist Jeffrey Tze-Fei Wong of the Hong Kong University of Science and Technology. “The 20 amino acids encoded by the universal genetic code were not happily and accidentally picked up by the early organisms. Instead, they were the end result of prolonged and ruthless mutations and selection,” he says.
Random alternatives
Evolution has been experimenting with random alternatives to the genetic code. “We would like to expand the genetic code in a direction that is solely determined by the chemist,” Liu says.
The Scripps group described its progress toward making unnatural organisms in two studies, one published in the April 29, 1999 Proceedings of the National Academy of Sciences and a second that appeared this year in the May 24 Journal of the American Chemistry Society.
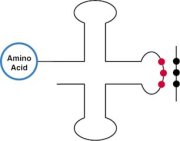
Introducing a new amino acid is quite a molecular engineering feat. First, the researchers had to manipulate molecules that are part of E. coli‘s protein-building machinery. These include transfer RNA (tRNA), which operates as a decoder, and the enzymes that add amino acids to each tRNA. Liu chose to work with molecules from yeast that select and add the amino acid glutamine.
Transfer RNAs have two business ends. At one, there’s the anticodon—the part of the molecule that reads the codon. The other end of the tRNA receives the corresponding amino acid from the tRNA-synthetase.
The researchers changed the anticodon in their new tRNA to match up with the UAG stop codon instead of the codon for glutamine. UAG is known among biochemists as the amber codon. Amber is the translation of Bernstein, the last name of one of the graduate students who first discovered the stop codon. Other scientists jokingly assigned the names ochre and opal to the stop codons UAA and UGA, respectively.
Normally, when a mutation that results in a new UAG codon occurs in a protein-coding sequence, the cell doesn’t complete the corresponding protein. The scientists’ altered tRNA, however, reads the amber codon as if it were glutamine and adds that amino acid, enabling the protein to grow to its normal length.
The Scripps researchers then turned to the second component of the protein-making machinery. They randomly mutated the gene for the synthetase molecule and examined the pool of variants that arose. Next, they selected those that add an amino acid other than glutamine to the tRNA. Some of the altered synthetases can hook up amino acids that scientists designed but that are not normally found in cells.
Liu put the tRNA and the entire library of synthetases into E. coli that had a mutation in a gene that makes the bacteria resistant to the antibiotic ampicillin. The mutation introduced the amber stop codon, making the protein so short as to be ineffective and the bacteria sensitive to the antibiotic.
Liu purposely chose to make this mutation at an innocuous site in the protein, so any amino acid—natural or otherwise—inserted there would produce a full-length, functional protein. He then fed the bacteria ampicillin and laboratory-produced amino acids. The only bacteria that survived were those that incorporated an amino acid—either a natural or an introduced one—at the location of the amber mutation.
At this point, the researchers didn’t know whether the cell was inserting a natural amino acid or one of the unnatural molecules, since both types of amino acids were available in the cell.
To find out what amino acid was added, the researchers put the altered tRNA and synthetase molecules from the bacteria that grew on ampicillin into another strain of E. coli. These bacteria already had an amber mutation in a gene that encodes barnase, a protein that kills bacteria. This time, Liu didn’t feed the bacteria any unnatural amino acids.
If the translation molecules corrected the amber mutation with a natural amino acid, the bacteria would make barnase and die. If they, however, needed to insert the unnatural molecules, now unavailable, the bacteria wouldn’t make barnase and would go on living. The experiment seems to have worked, but the researchers are still examining their most promising candidates.
Magliery is using the same strategy with a tyrosine tRNA and synthetase taken from Methanococcus jannaschii, a methane-producing archeabacterium that lives in extremely hot hydrothermal vents in the ocean. He, too, has promising candidates among his altered E. coli.
The Scripps researchers are also developing a system that employs four-base, instead of three-base, codons. “We’re certainly not the only people who have thought of this. In fact, nature thought of it,” says Magliery. Salmonella bacteria have devised a tRNA with a four-base anticodon, he says.
Japanese researchers at Okayama University have used a four-base codon to insert two unnatural amino acids into a protein in a test tube, they reported in the Dec. 29,1999 Journal of the American Chemical Society.
Ellington calls the Scripps group’s work on tRNA and synthetase “a monumental step forward” in trying to create an unnatural organism. He points out that in their work, unlike his own, there’s no evolution. To evolve and make full use of unnatural amino acids, he says, the organism would have to insert stop codons all over its genome.
That’s not likely to happen, says Ellington. More likely the organism would say, “‘No, thank you. I do not want your 21st amino acid anywhere else because it’s weird,'” he says.
Unnatural amino acids
In creating their Uncoli, the Texas researchers let evolution figure out how to deal with unnatural amino acids. Instead of engineering specific changes, the researchers used a strategy that might have gratified German philosopher Friedrich Nietzsche, who declared, “What does not destroy me makes me strong.”
Ellington gave the bacteria an amino acid—fluorotryptophan—that is normally a poison. He started with a mixture of fluorotryptophan and the natural amino acid tryptophan, then slowly decreased the concentration of tryptophan in the mixture. Some of the bacteria survived and grew in the brew with no tryptophan. They must be using fluorotryptophan in place of tryptophan, he and his coworkers surmise.
They are now trying to determine how the E. coli transformed into these Uncoli. Ellington says that he still has to prove that all of the tryptophans in all of the proteins have been replaced with fluorotryptophan before he will be convinced that his group has created an organism with an altered genetic code.
Although they may not have succeeded in making an unnatural organism yet, Schultz is encouraged by his group’s progress. “It’s a whole lot easier to push the door open wider than it is to get your foot in the door in the first place,” he says.
The method the Texas group used resembles the one that Wong used in 1983. Then working at the University of Toronto, he fed fluorotryptophan to a different bacterium, Bacillus subtilis.
Wong’s unnatural bacterium—given the modest designation HR15—made a surprising choice of poisons, says Ellington. In contrast to Uncoli, which is “a very grudging survivor” growing on flurotryptophan, HR15 grew happily on it. Not only did HR15 thrive on fluorotryptophan, it was poisoned by tryptophan. HR15 is not just a picky eater, but an entirely new type of life, Ellington says.
Wong agrees. “HR15 does represent a new form of life because the genetic code is the most basic attribute of living systems,” he says. He calls the alteration of the genetic code, “the ultimate test-tube evolution.”
“With other mutations, we are altering a macromolecule. With genetic code mutations, we are altering the whole organism,” Wong says.
The public has nothing to fear from these artificial organisms, says Schultz. HR15 has been stopped cold—it’s tucked away in a deep freezer at Wong’s lab. Any bacteria that escaped the lab would starve without the researchers feeding them the unusual amino acids.
Bioethicist Caplan dismisses any charges that the researchers are playing God. He says the scientists are “playing man” and doing what people do best—creating new things. “There’s nothing wrong, morally, with inventing things,” he adds.
*In the table in the article, the abbreviation for the newly introduced glutamine should be Gln not Glu. The figure wrongly gives Asn as the abbreviation for glutamine.