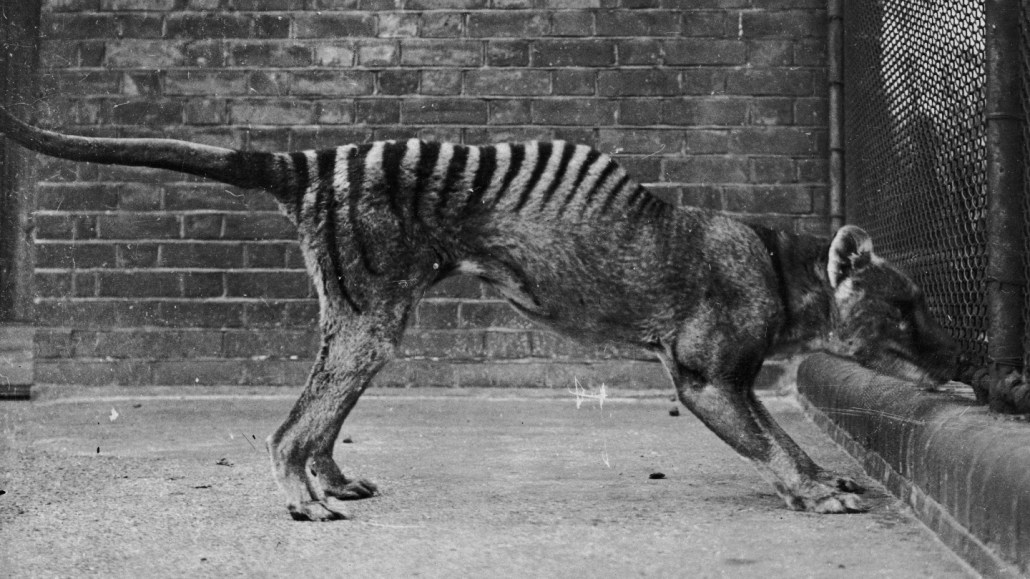
The Tasmanian tiger (one shown in captivity in 1930) went extinct after the last one died in a zoo in 1936. Researchers have now extracted RNA from one museum specimen.
Topical Press Agency/Stringer/Hulton Archive/Getty Images
For the first time, researchers have successfully extracted and decoded RNA from an extinct animal.
The thylacine, also known as the Tasmanian tiger, was a wolflike marsupial that went extinct after the last one died in a zoo in Hobart, Tasmania in 1936. Now a roughly 130-year-old museum specimen has yielded bits of RNA, the fragile molecules responsible for turning DNA’s genetic instructions into cellular functions, researchers report in the August Genome Research. The results shed new light on thylacine biology and may inform efforts to bring the marsupial back from extinction.
With dark stripes running over its tawny coat from its shoulders to its tail and jaws capable of opening more than 80 degrees, the thylacine (Thylacinus cynocephalus) was a striking animal. But the carnivores were no match for humans: As sheep farming proliferated in the 1800s in Tasmania — the home of the last remaining wild population of thylacine — the animals were frequently implicated in killing livestock. In the late 19th century, a bounty was established for every adult thylacine killed, and the animals were hunted nearly to extinction.
In recent years, researchers have mapped out the thylacine genetic blueprint, in addition to the genomes of other extinct animals like the woolly mammoth (SN: 2/17/21). But such investigations were all focused on DNA. Only RNA can reveal how an organism’s cells actually functioned, says Emilio Mármol-Sánchez, a geneticist at the Karolinska Institute in Stockholm. “You see the real biology of the cell.”
In 2020, Mármol-Sánchez and colleagues came across a thylacine specimen in storage at the Natural History Museum in Stockholm. “It was just there in a cupboard,” says Mármol-Sánchez, then at Stockholm University and the Center for Paleogenetics in Stockholm.
The team collected six small samples of skin and muscle from the desiccated animal. Back in the laboratory, the researchers ground each sample into a powder and added chemicals that isolated nucleotides, the building blocks of RNA. Next, the team used a computer algorithm to compare those strings of nucleotides, or sequences, with a database containing the genomes of thousands of animals, plants, fungi, bacteria and viruses — including the thylacine.
The team concluded that roughly 70 percent of the RNA sequences they found were reliably thylacine, with some contamination from human RNA since the thylacine specimen was repeatedly handled.
Their analysis revealed different protein-coding RNA molecules in their skin and muscle samples. That makes sense, Mármol-Sánchez says. “Muscle cells and skin cells serve quite different functions in the body.” For instance, the researchers pinpointed RNA molecules that coded cells to make slow-twitch muscle fiber, which helps with endurance.
The team also found over 250 thylacine-specific short RNA molecules known as microRNAs. These RNA sequences regulate cell functioning, Mármol-Sánchez says. “They’re the policemen of the cell.”
These are impressive results, says Andrew Pask, a developmental biologist at the University of Melbourne in Australia who was not involved in the research. Many researchers never even look for RNA, he says. “It’s much less stable than DNA.” And the findings are doubly impressive given that the specimen was stored at room temperature, Pask says, rather than in sterile or frozen conditions. (RNA has been previously extracted from samples of existing species preserved in alcohol or ice.) “It’s transformed the way that we look at museum and archive specimens.”
In the not-too-distant-future, Pask and other researchers are hoping to bring thylacine back to Tasmania. Their plan to de-extinct the animal involves modifying the genes of one of the thylacine’s closest living relatives, another marsupial called a fat-tailed dunnart (Sminthopsis crassicaudata). These new findings could very well inform that effort, Pask says, by revealing genes that controlled the animal’s attributes. “It’s a whole other layer of information.”






