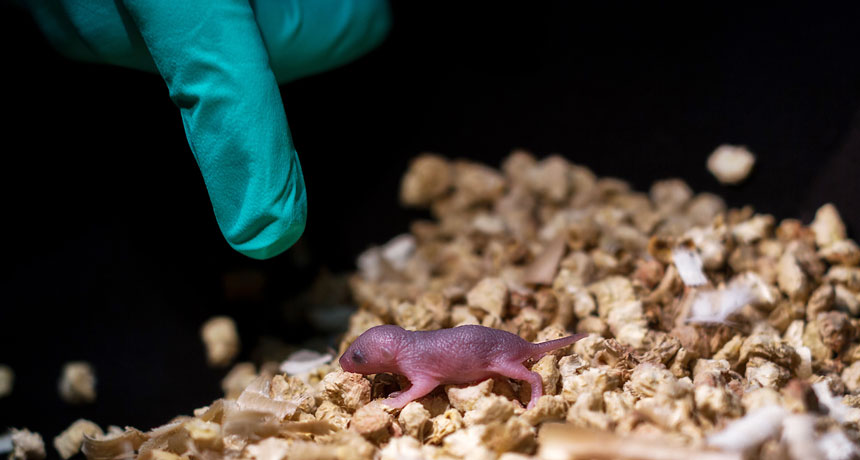
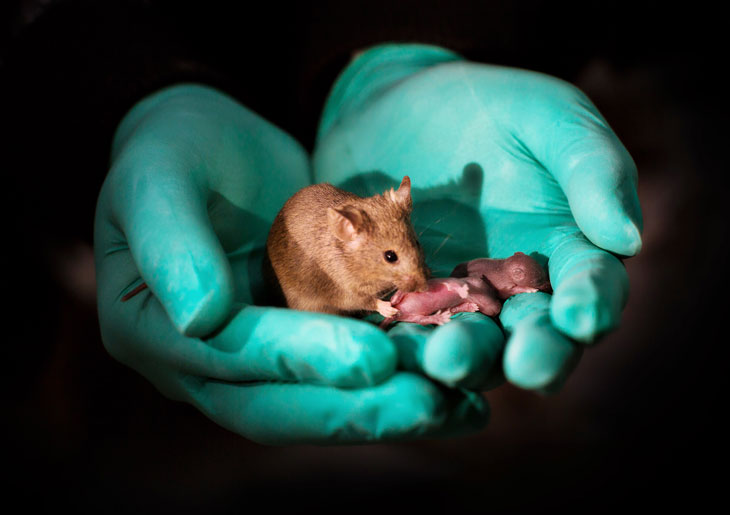
MY TWO DADS This mouse pup has two biological fathers and no mother. The mouse was produced in a lab from stem cells from two male mice. It did not survive to adulthood.
Leyun Wang
For the first time, researchers have created mice with two dads. No female contributed to the rodents’ genetic makeup.
This unusual reproduction took place in a lab where researchers gathered fathers’ stem cells, and used them to produce embryos that were implanted into surrogate mothers. The technique required scientists to edit the animals’ genes in order for the mice to mature enough to be born. Even so, mouse pups with only fathers died a few days after birth, researchers report October 11 in Cell Stem Cell. By contrast, previous research and this study have shown that some gene-edited mice with only mothers can survive to adulthood and have offspring of their own.
The researchers did the experiments to learn why mammals can reproduce only sexually — requiring two parents of the opposite sex — while other vertebrates, including turkeys, snakes and sharks, can sometimes reproduce with only one parent, says study coauthor Qi Zhou of the Chinese Academy of Sciences in Beijing. Females of those species can sometimes cause an unfertilized egg to produce offspring, a process called parthenogenesis.
Researchers have previously made zebrafish with only an individual father’s DNA. But no one before now has reported achieving male-only reproduction, or androgenesis, with mammals.
In the new work, mouse pups with two mothers were smaller than usual and had other abnormalities, the researchers found. Those deficits happened because some genes that the pups inherited from their mothers were imprinted, or marked with molecules known as methyl groups that are attached to DNA in spots near the gene. Imprinting may cause some genes to be more active and others less active.
So the researchers used a molecular scissors known as CRISPR/Cas9 to snip out three imprinted regions near important genes in stem cells that were then used to produce embryos. Of 210 of these embryos implanted in surrogate mothers, about 14 percent were born. Those gene-edited pups grew normally and became adults with normal fertility.
Making embryos from stem cells of two male mice wasn’t as successful.
To even get embryos made from two dads’ stem cells to form, the researchers had to snip out six imprinted pieces of DNA. Still, only 1.2 percent of 1,023 embryos with the six edits produced pups. Those pups were twice the size of normal pups and died soon after birth. Cutting out a seventh imprinted region resulted in pups of normal size, but only two pups lived more than 48 hours and neither survived to adulthood.
Still, the work is an important first step in understanding what the imprinted regions do during normal development, says B. Duygu Özpolat, a developmental biologist at the Marine Biological Laboratory in Woods Hole, Mass., who was not involved in the work.
That knowledge might help correct birth defects caused by imprinting errors, she says.
Making mammals from single sex parents might also help endangered species that have animals of only one sex left, Özpolat says. For instance, the last male northern white rhinoceros died earlier this year, leaving only two females (SN: 8/4/18, p. 8). Gene editing might help researchers bring white rhinos back by making all-female populations from lab grown stem cells.
“It might be too expensive, and might not work for every species, but it’s something,” Özpolat says. As for human same sex couples hoping to have a biological baby together, she says, “that’s the far future for the moment.”
Zhou adds that it could be too dangerous to try the technique in people. There’s no guarantee that the imprinted regions involved in mouse reproduction are the same ones involved in human reproduction. At any rate, he says, “to apply this technique into human is not one of our goals.”