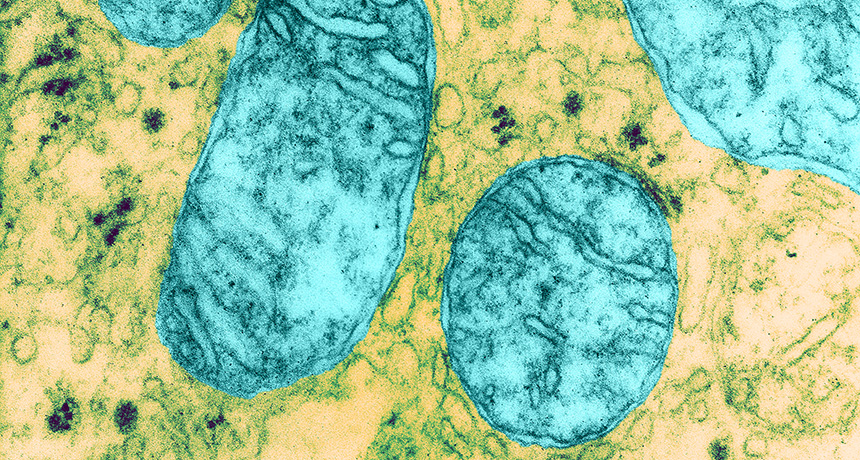
HEAVE HO Mitochondria (blue), energy-producing structures in cells, may get mutations that lead to diseases in people. Researchers have described a new genetic engineering technique that will eliminate mutant mitochondria from eggs and early embryos to prevent mitochondrial diseases from passing from mother to child.
Martin M. Rotker/Science Source