Let there be light
New technology illuminates neuronal conversations in the brain
In the beginning, the brain was a dark and shapeless void.
Then scientists deployed dyes, and lo, the intricate branching of brain cells called neurons was revealed. It was good but didn’t show which cells rubbed branches with others.
After a time, scientists brought forth electrodes and functional MRI machines to eavesdrop on neurons’ electrical chatter. It was good, but the message was hearsay. It could not show that any specific chitchat caused a particular behavior.
Then the scientists said let there be light, and a new age of neuroscience dawned. Now researchers create light-responsive molecules — or borrow them from microorganisms — to insert into animals’ neurons. And light shines upon the molecules, giving scientists dominion over the brain cells’ activity.
Harnessing light’s power has given birth to a burgeoning new field called optogenetics, which allows scientists to control neurons in freely moving animals. Although the technology is new, it is already beginning to illuminate some of the darkest corners of the brain, such as the connections that guide movement or make memories and the neuronal circuits that go haywire in depression, addiction or schizophrenia. What scientists learn from the light-aided experiments may lead to refinements of existing therapies or to new treatments for nervous system disorders.
After its debut in 2002, optogenetics went through a development period. Scientists had to demonstrate that the technology could change activity of brain cells and behavior in moving animals. Only in the last two years have light-driven experiments delivered unexpected results about how the brain and nervous system work, elucidating causes and effects.
“It took a few years to go from potential to fruition,” says neuroscientist Karl Deisseroth of Stanford University, a pioneer of the optogenetics movement. “We’ve turned a corner.” Now, more than 500 laboratories are using optogenetics to probe the brains of mice, fruit flies, zebra fish and nematodes, and even to probe human neurons growing in lab dishes, to “get to the neural code for complex things, such as reward,” he says.
Optogenetics may help neuroscience mature as a scientific discipline, says Gero Miesenböck, a neuroscientist at the University of Oxford in England and a founding father of the field. With the advent of optogenetics, he says, “neuroscience is now finally catching up to the widely held standards of proof in other fields of biology and chemistry to help establish causality.”
Light-responsive molecules used in optogenetics experiments have two basic modes. Some are neuron activators. When a specific wavelength of light shines on the cells engineered to carry these molecules, a channel opens and allows positively charged ions to flow into the cell. “This happens to be the neural code for ‘on,’” Deisseroth says. Other light-responsive molecules, when tickled with the correct wavelength of light, let negatively charged ions into the cell. The influx of negative ions silences neurons. Using combinations of the two types of molecules and different wavelengths of light, researchers can flip neurons on and off at will to find out how neurons interact with their neighbors.
For now, knowledge of those interactions is limited to small groups of neurons within more extensive brain circuits. But by flipping light-controlled switches, scientists may eventually construct a full diagram of the brain’s wiring.
“The most exciting application right now is the ability to control the activity, remotely and noninvasively, of neurons,” says Herwig Baier, a neuroscientist at the University of California, San Francisco. “It’s like a functional MRI, except it’s truly functional. You can really show causality.”
Functional MRI has helped researchers peer into living brains, revealing which areas of the brain are active (SN: 12/19/09, p. 16). But optogenetics allows scientists to manipulate neurons instead of just observing them. Scientists can identify specific brain circuits, such as those that help fruit flies sing love songs or give fish their tail swish, as well as those that push mice into addiction or cause them to collapse into depression.
Flipping the switch
In 2008, Miesenböck and colleague J. Dylan Clyne of Yale University reported in Cell that they had used light to manipulate a brain circuit that controls courtship behavior in the fruit fly Drosophila melanogaster. Researchers had already known that one form of a protein called fruitless is made in some neurons in male fruit fly brains and that those neurons help regulate the wing vibrations that create the flies’ mating songs.
This form of fruitless had been found in males but not females, and researchers think that the protein form helps wire males’ brains to sing courtship songs. Females, scientists had thought, may lack the brain circuit for creating the mating serenade.
Clyne and Miesenböck engineered fruit flies of both sexes to carry a light-responsive protein in the neurons that make this fruitless in male flies. A pulse of UV light activated the neurons, causing males to immediately beat their wings. But female flies began to sing sultry music (to a fruit fly), too. The result indicates that male and female flies have the same underlying brain circuitry, a surprise to the researchers.
“We really had no inkling that there is a unisex structure that you can switch to male or female behavior,” Miesenböck says. “It’s really an elegant solution.”
Though Miesenböck doesn’t much care about the love lives of fruit flies, studying the insects may help him figure out how the brain ticks.
He and his colleagues are using the technology for other basic biology problems as well. Memory studies, for instance, usually focus on the effect of disrupting a particular gene or use psychological tests to try to determine how memories are made. But neither of those types of experiment reveals which neuronal circuits are activated during the memory-making process.
“These approaches, I feel, leave the black box pretty firmly shut,” Miesenböck says. Genetic experiments that disrupt a gene and shut down brain cell activity aren’t as informative as finding out what happens when a particular circuit is activated, he says. “There are many ways you can break something, but often there’s only one way to make it work.”
His team plugged in light-activated molecules to create false smell memories in fruit flies. Formation of smell memories is known to require dopamine, which interacts with cells in brain structures known as mushroom bodies. But researchers didn’t know exactly which neurons make the dopamine, or the identity of the cells within the mushroom bodies that receive the chemical’s message.
By engineering different sets of neurons to respond to light and turning those neurons on at different times, Miesenböck’s team tracked the source of particular smell memories to a cluster of 12 dopamine-producing cells known as the PPL1 neurons. By triggering those cells, researchers created an aversive memory akin to that created by pairing an odor with a shock, the researchers reported in the Oct. 16 Cell.
The experiments identified the signal that teaches flies not to like a particular smell but didn’t uncover the source of the signal, Miesenböck says. “We would like to meet the teacher.” Further light-guided experiments might eventually garner such an introduction.
Optogenetics is helping scientists more precisely probe the activity of nerve cells in the spinal cord, too.
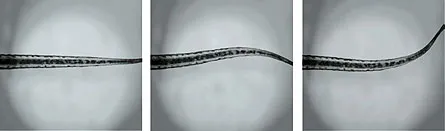
Baier and colleagues recently got a bright idea about the raison d’être of some spinal cord neurons with a name but no known function. Kolmer-Agduhr cells are found in the spinal cords of all vertebrates, but no one knew why the cells are there or what they do. Baier’s team inserted a light-activated molecular switch, known as LiGluR, into the Kolmer-Agduhr cells in zebra fish and then flipped on a UV light.
“The results were really astounding,” Baier says. When the light came on, the fish’s tails began to swish back and forth in swimming mode. With light activation, the cells still caused the swimming movements even when the connection between the brain and spinal cord was severed, the team reported September 17 in Nature. “All together, these observations show that the forward swim can be attributed specifically to the activation of the Kolmer-Agduhr cells,” the researchers wrote.
But the experiments showed that the cells are not involved in all types of movement. For example, the cells didn’t help when the fish sensed a touch and bent their tails in a C-curve to escape predators. Now Baier and his colleagues are probing zebra fish brains with light to dissect the pathways that control motion. Eventually, such experiments may help lead to a better understanding of movement disorders in people.
Light shed on brain disorders
Deisseroth’s group has already illuminated brain circuitry involved in Parkinson’s disease (SN: 4/11/09, p. 11). The team found that stimulating certain neurons in the motor cortex could quell Parkinson’s-like symptoms in mice. The discovery, reported online last March in Science, could lead to the development of less invasive treatments for the disorder than the currently used deep-brain stimulation.
Other brain disorders, including mental illnesses, may arise when certain circuits misfire. Scientists are tracking down these short-circuits by following optogenetically illuminated pathways. Previous work had shown that chronic social stress — the equivalent to being constantly bullied in humans — can make mice depressed. That depression is associated with lower activity levels of certain genes in the part of the brain called the medial prefrontal cortex, as well as other brain changes.
Researchers at Mount Sinai School of Medicine in New York City engineered mice so that their brain cells make a light-activated protein found in a single-celled alga called Chlamydomonas reinhardtii. The protein, channelrhodopsin-2, responds to certain wavelengths of blue light by letting in positively charged ions and turning neurons on. When housed with bigger, tougher mouse bullies, the mice became antisocial, a sign of depression. But shining a blue light on the medial prefrontal cortex turned on cells making channelrhodopsin-2, and that stimulation reversed the depression about as well as an antidepressant drug, Herbert Covington of Mount Sinai reported in Chicago in October 2009 at the annual meeting of the Society for Neuroscience. The work may help scientists find the cells where changes that lead to depression first occur.
On the flip side of depression’s darkness is the glow of reward. No one has precisely mapped how the reward system is wired, but when it goes awry, it’s a major player in addiction. Garret Stuber of UC San Francisco and colleagues are tracing reward circuits in the brains of mice by following the light.
Stuber’s team injected a virus carrying channelrhodopsin-2 into the amygdala, an emotion-processing center, in the brains of mice. The virus can travel in the brain along fibers that connect the amygdala to other brain regions, including the nucleus accumbens, a part of the brain previously shown to be important in addiction. As the virus spreads, it infects other cells in the region the researchers are looking at.
Shining a blue light on the nucleus accumbens activated connections to the amygdala, rewarding the mice. Mice trained to poke their noses into a hole to trigger a pulse of light on the nucleus accumbens kept going back again and again for another flash, indicating that the light flashes caused a reward response. But mice that got a flash of light to stimulate connections between the nucleus accumbens and the prefrontal cortex didn’t seem to have a rewarding experience, Stuber reported at the neuroscience meeting. That suggests that the connections between the prefrontal cortex and nucleus accumbens are not involved in this reward circuitry.
Optogenetics can also help scientists learn more about circuitry associated with normal brain functions, says Michael Häusser of University College London. He and his colleagues are investigating a long-standing debate in neuroscience about how the brain recalls memories.
Learning is thought to activate networks of neurons, and scientists think activating subsets of the cells in the networks may reactivate a memory. Before optogenetics, there was no way to directly test that hypothesis.
Häusser’s group presented preliminary, unpublished evidence supporting the idea at the neuroscience meeting. The team injected a piece of DNA into the hippocampus of mice that would produce channelrhodopsin-2 wherever neuronal remodeling associated with learning occurred. The hippocampus is an area of the brain known to be important in learning and memory.
The next day, the mice were trained to fear a shock on the foot. Mice that learned to connect an audio tone and the shock froze, a known response to fear, when they heard the tone, even if no shock followed.
Light shining into the hippocampus could also activate the cells that made the mice freeze, indicating that those cells were involved in learning to fear the shock. The researchers also tested whether activating any cells in the hippocampus could cause the fear response. Only those cells involved in the initial learning could make mice freeze. The researchers further found that activating just 100 to 200 cells is enough to reproduce the behavior, suggesting that the reactivation theory could be correct.
Miesenböck thinks optogenetics may help settle this reactivation debate and answer other basic questions about brain biology, such as whether the precise timing of each spike of electricity a neuron sends out is important, or neighboring cells listen only to the average pattern of activity. Optogenetics isn’t yet precise enough to answer that question and has some other limitations, Miesenböck says. He outlined the technique’s strengths and weaknesses in the Oct. 16 Science.
“Optogenetic technology, despite all of its refinement, is not able to control activity of individual members of a group of neurons,” he says.
And for all its power, it’s not intended to be a therapeutic tool; that would require gene therapy and brain surgery. But optogenetics is helping scientists discover things about the brain and nervous system that no one ever knew before. And, for that reason, it is good.
Sign up for our newsletter
We summarize the week's scientific breakthroughs every Thursday.