Microbes Fire an Oozie: Slime engines may push bacteria along
Threads of goop oozing from tiny nozzles in the cell walls of some bacteria exert sufficient force to nudge the creatures along, a new study finds. This result may solve a decades-old puzzle about bacterial motion.
Many bacteria get around by gliding on a film of slime. Microbiologists had long observed that those microbes, when traveling in groups, shoot out filaments, or pili, that anchor to the creatures’ surroundings. The bacteria then jerk themselves along by these cords. However, when the same bacteria wander on their own, they cruise smoothly with no evidence of pili or any other mechanism for movement. “People have been looking to understand this for 30 to 40 years,” says Patricia L. Hartzell of the University of Idaho in Moscow.
Four years ago, microscopist Egbert Hoiczyk, now at Rockefeller University in New York, and his coworkers discovered minuscule nozzles in the walls of the microbes called cyanobacteria. The barrel-shaped pores excreted slime at a rate proportional to the speed at which the creatures glided. Perhaps the slime itself was pushing the creatures along, they proposed.
That seemed unlikely, however, because it would require a large amount of slime. The cyanobacteria “would basically be spending all their time making this material in order to move,” Hartzell notes.
Now, Hoiczyk, with George F. Oster and Charles W. Wolgemuth of the University of California, Berkeley and Dale Kaiser of Stanford University, offer a new strategy by which bacteria can use slime to glide along without bleeding themselves dry. The team presented its findings this week at a meeting of the American Physical Society in Indianapolis. A report on the new work also appears in the March 5 Current Biology.
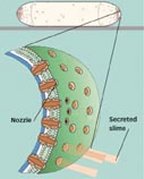
The researchers worked with Myxococcus xanthus, a type of bacterium not closely related to cyanobacteria. Examination with an electron microscope revealed that slime emerges in thin bands from the ends of the bacteria. Hoiczyk and his colleagues counted about 250 nozzles encircling each blunt end of this rod-shaped myxobacterium.
Previous research has indicated that bacterial slime is a polyelectrolyte gel, which expands in water. Mathematically analyzing nozzle action, the team finds that a slime engine with such a substance is feasible. A familiar example of a polyelectrolyte gel, Oster says, is the mucin granules secreted in a person’s nose. When those granules encounter water, they swell to form viscous mucus.
When bacteria excrete some of their dehydrated gel into the nozzles, they may trigger what Oster calls “snot guns.” The gel particles swell dramatically upon encountering water from outside the cell and then erupt out the nozzles’ open ends. By pressing against external surfaces, the slimy protrusions may drive the bacterium.
In the gun hypothesis, a bacterium produces lots of slime for propulsion from only a small amount of gel–thereby conserving its resources for other activities, the scientists say. Moreover, they calculate, the thrust from just 50 nozzles is sufficient to move both cyanobacteria and myxobacteria at their observed gliding speeds.
Hartzell calls the snot gun “a neat idea,” and she speculates that it’s also used in other microorganisms. However, she’s not convinced that the nozzles are as concentrated at the cell ends as Hoiczyk has reported.
It “seems likely” that some bacteria use slime propulsion, comments Howard C. Berg of Harvard University. However, many aspects of bacterial motion still lack an explanation, he says.