Mining the Mouse
A rodent's DNA sheds light on the human genome
In 1906, a descendant of Paul Revere named Clarence Cook Little was pursuing studies in the new discipline of genetics while attending Harvard University. One of his professors challenged him to do a project on the inheritance of coat color in mice. As part of the effort, Little mated brothers to sisters and created the very first inbred strains of mice. This reduced the variation among the mouse genes and made it easier for scientists to study inheritance.
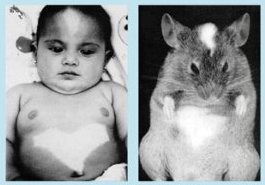

Little rode his pioneering mouse work to prominence, becoming the president of the University of Maine and later of the University of Michigan. In 1929, he founded a facility in Bar Harbor, Maine, devoted to using mice to study cancer and other aspects of mammalian biology.
Later that year, however, the stock market collapsed, and funds for research dried up. Little and his colleagues at the Maine center raised money by selling inbred strains of mice to other scientists. That tradition continues today. The center, now known as Jackson Laboratory, houses and sells thousands of unusual strains of mice, such as abnormally obese or cancer-prone ones.
Little, who died in 1971, would be surprised to see the current scale of Jackson Laboratory. However, the geneticist would be even more stunned by the progress in mouse genetics over the past few years. Using an inbred strain that Little originally created, one known as C57BL, an international consortium of scientists has deciphered nearly the entire DNA sequence of the mouse. This makes the rodent only the second mammal, after people, to have its full DNA sequence, or genome, revealed.
Two months ago, the fruits of that accomplishment started to become clear. In a series of reports in the Dec. 5, 2002 Nature, biologists disclosed the results of their initial studies of the mouse genome and its comparison with the human genome. The mouse seems to have almost the exact same set of protein-coding genes that people do. In addition, when the scientists compared mouse and human DNA sequences that don’t encode proteins, they found many more shared sequences than they had expected.
Researchers contend that insights gleaned from the mouse genome will ultimately have a profound impact on biomedical research. “It’s clear that the mouse is a Rosetta stone for understanding human biology,” says Robert Waterston of Washington University School of Medicine in St. Louis.
“With two genomes, we can begin to pick out what matters,” adds Eric S. Lander of the Whitehead Institute of Biomedical Research in Cambridge, Mass. “We’ve been missing much of the story.”
Shuffling around
About 75 million years ago, a ratlike animal, the last common ancestor of mice and people, roamed the planet alongside dinosaurs. Since that period, evolution has scrambled that creature’s genetic code enough to produce two extraordinarily different species: Homo sapiens and Mus musculus, the common laboratory mouse.
A superficial look at the human and mouse genome reflects the huge gulf between the mouse and humankind. People have 23 pairs of chromosomes, while mice have only 20, for example. One measure of genome size–the number of base pairs, or subunits of DNA–displays a similar gap. The mouse genome has about 2.5 billion base pairs, significantly less than the 2.9 billion base pairs that make up the human genetic code, according to Lander, Waterston, and their dozens of colleagues. That team sequenced the mouse genome over the past few years, as has Celera, a biotech firm in Rockville, Md. However, Celera supplies its data only to paying customers and has published just one report on one mouse chromosome.
The number of chromosomes or base pairs by itself doesn’t offer much insight into an animal’s biology. For instance, closely related species can pack the same amount of DNA into very different numbers of chromosomes. And the mouse’s smaller number of base pairs may simply stem from that animal’s ridding its genome more effectively of so-called junk DNA sequences than humans did.
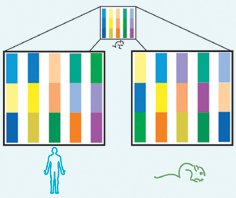
Indeed, a close look at the two genomes reveals striking similarities. The order of genes on each species’ chromosomes has been so well preserved that researchers can line up some 350 blocks of mouse genes–representing more than 90 percent of the genome–with areas in the human genome.
Evolution may have shuffled those segments quite a bit, but the correspondence holds. “If you know where you are in the human genome, you know where you are in the mouse genome, and vice versa,” says Lander. It’s like two books that have the same chapters but in different orders, he notes. Imagine if three sections of human chromosome 11 showed up as two areas on mouse chromosome 3 and one on mouse chromosome 5. From such data, geneticists can reconstruct the blocks of genes that probably existed in the chromosomes of the two species’ common ancestor.
Mice and people also possess a similar complement of genes, say the researchers. The total number of genes used by people has been debated ever since the initial analyses of the human genome sequence 2 years ago indicated that people have just 30,000 to 40,000 genes, rather than the 75,000 to 100,000 genes that many scientists had predicted. Much of the debate centers on the definition of a gene, a surprisingly complicated issue because a cell may read the same DNA sequence in different ways to produce distinct proteins.
Today, the scientists analyzing the mouse genome estimate that the animal, like a person, has about 30,000 genes. The mouse genome has already helped scientists find more than 1,000 new human genes. Francis Collins, director of the National Human Genome Research Institute in Bethesda, Md., points to the recent discovery of a gene involved in fat metabolism that may have a role in heart disease. This gene was found in the human genome only after investigators detected its mouse version, he says.
In other cases, scientists have used the mouse data to determine that a given human DNA sequence isn’t a gene. “The mouse genome is helping us clean up a lot of noise,” Lander says.
He and the other investigators who sequenced the mouse genome report that some 80 percent of mouse genes have a single counterpart in the human genome, while about 20 percent have multiple versions in people.
“Essentially, every mouse gene has a human [version],” says Lander.
That’s not to say researchers haven’t found significant differences between the two genomes. Of the mouse genes identified so far, there are 300 that don’t have a recognizable human copy. Moreover, mice have many more copies of certain genes involved in reproduction, immunity, and olfaction, for example. The greater number of working genes devoted to the proteins that detect scents may reflect that rodents have a greater dependence on their sense of smell than people do (SN: 5/6/00, p. 298: Disabled genes dull sense of smell).
Share and share alike
The biggest surprise emerging from the ongoing comparison of the mouse and human genomes is that they share many DNA sequences that don’t encode proteins. Slightly less than half of the shared DNA sequences don’t seem to encode proteins, and their function remains largely a mystery.
“What do they do? We don’t know. That’s what’s exciting,” says Lander, who jokes that he and other geneticists are “extending our ignorance” with their studies of the mouse genome.
Evolution is the great experimentalist, notes Landers, and it doesn’t preserve DNA sequences unless they provide vital functions for an animal. “Evolution’s job is to knock things out and see how it works,” he says.
Scientists suspect that most of this unexplained conserved DNA somehow regulates the activity of protein-coding genes. Dramatic differences in gene regulation probably explain how a similar set of genes can produce either a mouse or a person, they say.
Some of the shared DNA, however, appears to encode RNA strands rather than proteins as its end product. Just within the past few years, biologists have begun to realize that RNA strands of varying sizes may have unexpected roles in cells (SN: 1/12/02, p. 24: Biological Dark Matter). In its year-end issue, the journal Science called the growing appreciation of so-called RNA genes the most important scientific breakthrough of 2002.
Another mystery emerging from the mouse genome centers on the speed with which its DNA mutates. The mouse has “a rapidly changing genome, changing under forces we understand only poorly,” says Waterston.
Over the 75 million years since human and mouse ancestors diverged, the mouse genome has by some measures accumulated mutations at twice the rate of the human genome. More recently, says Waterston, the mutation rate of mice seems to have sped up to five times as fast as that of the human genome, he says. Moreover, the mutations seem to accumulate at different rates in various parts of a mouse chromosome.
Betting on 21
Several of the reports in the Dec. 5, 2002 Nature illustrate how the marriage of the mouse and human genomes may illuminate aspects of human health. For example, two research groups tallied the list of genes on a person’s chromosome 21 and examined when and where in developing mouse embryos the rodent versions of the genes are active.
There’s a practical reason why both teams focused on chromosome 21. About 1 in 700 newborns has three instead of two copies of this chromosome and so develops Down syndrome, the most common form of mental retardation.
Because the disorder seems to involve many genes, scientists have struggled to determine which ones are responsible for the physical and mental features of the disorder.
“Finding out when and where each chromosome 21 gene is expressed during development is a crucial step toward understanding the syndrome,” notes Roger H. Reeves of Johns Hopkins University School of Medicine in Baltimore.
Investigators at the Sanger Institute in Hinxton, England, have even more ambitious plans. They intend to document the pattern of activity of every mouse gene.
And researchers there and elsewhere have proposed creating so-called knockout mice–rodents in which a single gene has been deactivated–for all 30,000 rodent genes. The mutant mice should reveal which genes are crucial to mammalian development and to the health of the adult animal.
Appropriately, the laboratory that Clarence Cook Little created is among the groups entertaining the idea of such a massive project. “It’s not unrealistic,” says Rick Woychik, director of Jackson Laboratory. “It’s a resource that would be extremely valuable.”
****************
If you have a comment on this article that you would like considered for publication in Science News, please send it to editors@sciencenews.org.