Octopuses and squid are masters of RNA editing while leaving DNA intact
These changes could explain the intelligence and flexibility of shell-less cephalopods

Octopuses and other shell-less cephalopods commonly edit their RNA without changing their DNA. Scientists don’t yet know why.
WRANGEL/ISTOCK/GETTY IMAGES PLUS
Many writers grouse when an editor makes a change in a story, but the consequences of changing a single word usually aren’t that dire.
Not so with genetic instructions for making proteins. Even a small change can prevent a protein from doing its job properly, with possibly deadly consequences. Only occasionally is a change beneficial. It seems wisest to preserve genetic instructions as they are written. Unless you’re an octopus.
Octopuses are like aliens living among us — they do a lot of things differently from land animals, or even other sea creatures. Their flexible tentacles taste what they touch and have minds of their own. Octopuses’ eyes are color-blind, but their skin can detect light on its own (SN: 6/27/15, p. 10). They are masters of disguise, changing color and skin textures to blend into their surroundings or scare off rivals. And to a greater extent than most creatures, octopuses squirt the molecular equivalent of red ink over their genetic instructions with astounding abandon, like a copy editor run amok.
These edits modify RNA, the molecule used to translate information from the genetic blueprint stored in DNA, while leaving the DNA unaltered.
Scientists don’t yet know for sure why octopuses, and other shell-less cephalopods including squid and cuttlefish, are such prolific editors. Researchers are debating whether this form of genetic editing gave cephalopods an evolutionary leg (or tentacle) up or whether the editing is just a sometimes useful accident. Scientists are also probing what consequences the RNA alterations may have under various conditions. Some evidence suggests editing may give cephalopods some of their smarts but could come at the cost of holding back evolution in their DNA (SN: 4/29/17, p. 6).
“These animals are just magical,” says Caroline Albertin, a comparative developmental biologist at the Marine Biological Laboratory in Woods Hole, Mass. “They have all sorts of different solutions to living in the world they come from.” RNA editing may help give the creatures vast numbers of solutions for problems they may face.
How cephalopods modify their RNA
Molecular biology’s central dogma holds that instructions for building an organism are contained in DNA. Cells copy those instructions into messenger RNAs, or mRNAs. Then, cellular machinery called ribosomes read the mRNAs to build proteins by stringing amino acids together. Most of the time, the protein’s composition conforms to the DNA template for the protein’s sequence of amino acids.
But RNA editing can cause divergences from the DNA instructions, creating some proteins that have different amino acids than specified by the DNA.
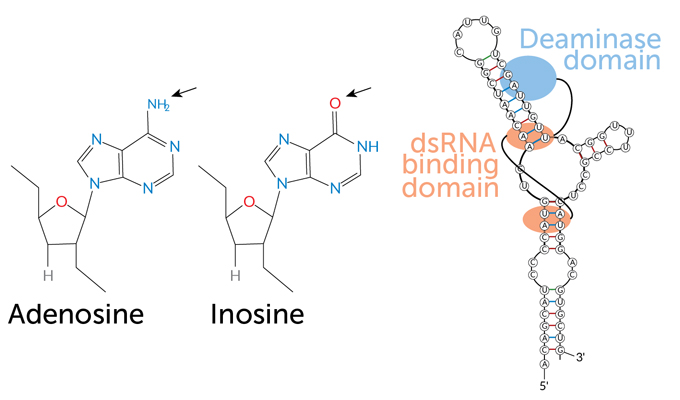
Editing chemically modifies one of RNA’s four building blocks, or bases. Those bases are often referred to by the first letters of their names: A, C, G and U, for adenine, cytosine, guanine and uracil (RNA’s version of the DNA base thymine). In an RNA molecule, the bases are linked to sugars; the adenine-sugar unit, for instance, is referred to as adenosine.
There are many ways to edit RNA letters. Cephalopods excel at a type of editing known as adenosine to inosine, or A-to-I, editing. This happens when an enzyme called ADAR2 strips a nitrogen and two hydrogen atoms off adenosine (the A). That chemical peel turns adenosine into inosine (I).
Ribosomes read inosine as guanine instead of adenine. Sometimes that switch has no effect on the resulting protein’s chain of amino acids. But in some cases, having a G where an A should be results in a different amino acid being inserted into the protein. Such protein-altering RNA editing is called RNA recoding.
Soft-bodied cephalopods have embraced RNA recoding with all of their arms while even closely related species are more tentative about accepting rewrites, Albertin says. “Other mollusks don’t seem to do it” to the same extent.
RNA editing isn’t limited to creatures of the deep. Almost every multicellular organism has one or more RNA editing enzymes called ADAR enzymes, short for “adenosine deaminase that acts on RNA,” says Joshua Rosenthal, a molecular neurobiologist also at the Marine Biological Laboratory.
Cephalopods have two ADAR enzymes. Humans have versions of them, too. “In our brains, we edit a ton of RNA. We do it a lot,” Rosenthal says. Over the last decade, scientists have discovered millions of places in human RNAs where editing occurs.
But those edits rarely change the amino acids in a protein. For instance, Eli Eisenberg of Tel Aviv University and colleagues identified more than 4.6 million editing sites in human RNAs. Of those, only 1,517 recode proteins, the researchers reported last year in Nature Communications. Of those recoding sites, up to 835 are shared with other mammals, suggesting that evolutionary forces preserved editing at those locations.
How does RNA editing work?
In a common form of RNA editing, an adenosine becomes an inosine through a reaction that removes an amino group and replaces it with an oxygen (arrows). The illustration shows an ADAR enzyme attaching to a double-stranded RNA at the “dsRNA binding domain.” The region of the enzyme that will interact to cause the reaction, the “deaminase domain,” is positioned near the adenosine that will become an inosine.
Cephalopods take RNA recoding to a whole new level, Albertin says. Longfin squid (Doryteuthis pealeii) have 57,108 recoding sites, Rosenthal, Eisenberg and colleagues reported in 2015 in eLife. Since then, the researchers have examined multiple species of octopus, squid and cuttlefish, each time finding tens of thousands of recoding sites.
Soft-bodied, or coleoid, cephalopods may have more opportunities for editing than other animals because of where at least one of the ADAR enzymes, ADAR2, is located in the cell. Most animals edit RNAs in the nucleus — the compartment where DNA is stored and copied into RNA — before sending the messages out to meet up with ribosomes. But cephalopods also have the enzymes in the cytoplasm, the cells’ jellylike guts, Rosenthal and colleagues discovered (SN: 4/25/20, p. 10).
Having editing enzymes in two locales doesn’t fully explain why cephalopods’ RNA recoding so far outstrips that of humans and other animals. Nor does it explain the patterns of editing scientists have uncovered.
RNA editing may give cephalopods flexibility
Editing isn’t an all-or-nothing proposition. Rarely are all copies of an RNA in a cell edited. It’s much more common for some percentage of RNAs to be edited while the rest retain their original information. The percentage, or frequency, of editing can vary widely from RNA to RNA or between cells or tissues, and may depend on water temperature or other conditions. In longfin squid, most RNA editing sites were edited 2 percent or less of the time, Albertin and colleagues reported last year in Nature Communications. But the researchers also found more than 205,000 sites that were edited 25 percent of the time or more.
In most of a cephalopod’s body, RNA editing doesn’t often affect the makeup of proteins. But in the nervous system, it’s a different story. In longfin squids’ nervous systems, 70 percent of edits in protein-producing RNAs recode proteins. And RNAs in the nervous system of the California two-spot octopus (Octopus bimaculoides) are recoded three to six times as often as in other organs or tissues.

Some mRNAs have multiple edit sites that alter amino acids in the proteins the mRNAs encode. In the longfin squid’s nervous system, for instance, 27 percent of mRNAs have three or more recoding sites. Some contain 10 or more such sites. Combinations of those editing sites could result in multiple versions of a protein being made in a cell.
Having a wide selection of proteins may give cephalopods “more flexibility in responding to the environment,” Albertin says, “or give you a variety of solutions to the problem in front of you.” In the nervous system, RNA editing might contribute to flexibility in thinking, which could help explain why octopuses can unlock cages or use tools, some researchers think. Editing could be an easy way to create one or more versions of a protein in the nervous system and different ones in the rest of the body, Albertin says.
When humans and other vertebrates have different versions of a protein, it often comes from having multiple copies of a gene. Doubling, tripling or quadrupling copies of a gene “results in a whole genetic playground to allow genes to go off and do different functions,” Albertin says. But cephalopods tend not to duplicate genes. Instead, their innovations come from editing.
And there is a lot of room for innovation. In squid, mRNAs for building the alpha-spectrin protein have 242 recoding sites. All the combinations of edited and unedited sites theoretically could create up to 7 x 1072 forms of the protein, Rosenthal and Eisenberg report in this year’s issue of Annual Review of Animal Biosciences. “To put this number in perspective,” the researchers wrote, “suffice it to say that it dwarfs the number of all alpha-spectrin molecules (or, for that matter, all protein molecules) synthesized in all cells of all the squids that have ever lived on our planet since the dawn of time.”
That incredible level of complexity would be possible only if every site were independent, says Kavita Rangan, a molecular biologist at the University of California, San Diego. Rangan has been studying RNA recoding in California market squid (Doryteuthis opalescens) and in longfin squid. Water temperature triggers the squid to recode motor proteins called kinesins that move cargo inside cells.
In longfin squid, the mRNA that produces kinesin-1 has 14 recoding sites, Rangan has found. She examined mRNAs from the optic lobe — the part of the brain that processes visual information — and from the stellate ganglion, a collection of nerves involved in generating the muscle contractions that produce jets of water to propel the squid.
Each tissue made several versions of the protein. But certain sites tended to be edited together, Rangan and Samara Reck-Peterson, also of UC San Diego, reported last September in a preprint posted online at bioRxiv.org. Their data suggest that editing of some sites is coordinated and “very strongly rejects the idea that editing is independent,” Rangan says. “The frequency of the combos that we see don’t match if every site was edited independently.”
Yoking editing sites may prevent squid and other cephalopods from reaching the pinnacles of complexity that they’re theoretically capable of. Still, RNA editing provides cephalopods a way to try out many versions of a protein without getting locked into a permanent change in DNA, Rangan says.
That lack of commitment puzzles Jianzhi Zhang, an evolutionary geneticist at the University of Michigan in Ann Arbor. “It doesn’t make sense to me,” he says. “If you want a particular amino acid in a protein, you should change the DNA. Why do you change the RNA?”
Is there evolutionary value to RNA editing?
Perhaps RNA editing provides some evolutionary advantage. To test that idea, Zhang and then–graduate student Daohan Jiang compared “synonymous” sites, where edits do not change amino acids, with “nonsynonymous” sites where recoding happens. Since synonymous edits don’t change amino acids, the researchers considered those edits to be neutral as far as evolution is concerned. In humans, recoding, or nonsynonymous editing, happens at fewer sites than synonymous editing, and the percentage of RNA molecules that are edited is lower than at synonymous sites.
“If we assume synonymous editing is just like noise that happens in the cell, and nonsynonymous editing is less frequent and [at a] lower level, that suggests nonsynonymous editing is actually harmful,” Zhang says. Even though recoding in cephalopods happens much more frequently than for humans, in most cases, recoding is not advantageous, or adaptive, for cephalopods, the researchers argued in 2019 in Nature Communications.
There are a few shared sites where octopuses, squid and cuttlefish all recode their RNAs, the researchers found, suggesting the recoding is useful in those instances. But this is a small fraction of editing sites. A few other sites that are edited in one species of cephalopod but not others were also adaptive, Zhang and Jiang found.
If it’s not all that helpful, why have cephalopods persisted with RNA recoding for hundreds of millions of years? RNA editing may stick around not because it is adaptive, but because it is addictive, Zhang says.
He and Jiang proposed a harm-permitting model (that is, a situation that permits harmful changes to DNA). Imagine, he says, a situation in which a G (guanine) in an organism’s DNA gets mutated to an A (adenine). If that mutation leads to a harmful amino acid change in a protein, natural selection should weed out individuals that carry that mutation. But if, by chance, the organism has RNA editing, the mistake in the DNA might be corrected by editing RNA, essentially changing the A back to G. If the protein is essential for life, then the RNA would have to be edited at high levels so that nearly every copy is corrected.
When that happens, “You’re locked into the system,” Zhang says. Now the organism is dependent on RNA editing machinery. “It cannot be lost, because you will require the A to be edited back to G for survival, so the editing will be kept at high levels.… In the beginning you really didn’t need it, but after you got it, you became addicted.”
Zhang argues that that sort of editing is neutral, not adaptive. But other research suggests RNA editing can be adaptive.
RNA editing may work as a transition phase, letting organisms try out a switch from adenine to guanine without making a permanent change in their DNA. Over the course of evolution, sites where adenines are recoded in RNA in one cephalopod species are more likely than unedited adenines to be replaced with guanine in the DNA of one or more related species, researchers reported in 2020 in PeerJ. And for heavily edited sites, evolution across cephalopods seems to favor a transition from A to G in DNA (rather than to cytosine or thymine, the other two DNA building blocks). That favors the idea that editing can be adaptive.
Other recent work by Rosenthal and colleagues, which examined A-to-G replacements in different species, suggests that having an editable A is an evolutionary boon over an uneditable A or a hardwired G.
How common is RNA recoding?
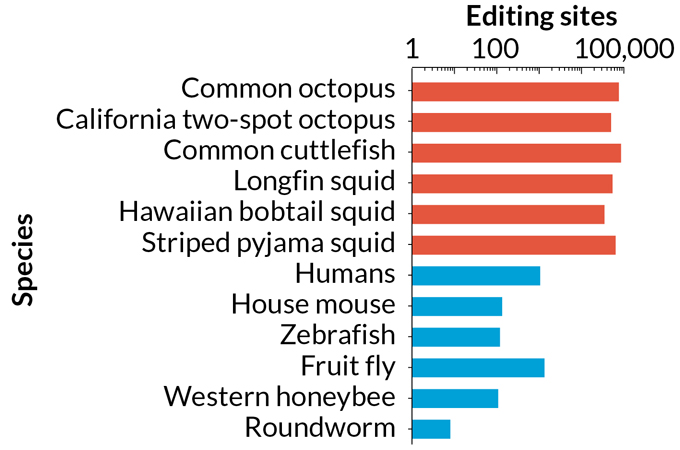
Soft-bodied cephalopod species including octopuses, squid and cuttlefish recode RNA in their nervous systems at tens of thousands of sites, compared with about a thousand or fewer sites in humans, mice, fruit flies and other animal species. Though scientists have been documenting the number of editing sites, they will need new tools to directly test how recoding influences cephalopod biology.
Number of RNA recoding sites across animals
There are a lot of open questions
Evidence for and against RNA recoding’s evolutionary value has come mainly from examining the total genetic makeup, or genomes, of various cephalopod species. But scientists would like to directly test whether recoded RNAs have an effect on cephalopod biology. Doing that will require some new tools and creative thinking.
Rangan tested synthetic versions of squid motor proteins and found that two edited versions that squid make in the cold moved slower but traveled farther along protein tracks called microtubules than unedited proteins did. But that’s in artificial laboratory conditions on microscope slides. To understand what is happening in cells, Rangan says, she would like to be able to grow squid cells in lab dishes. Right now, she has to take tissue directly from the squid and can only get snapshots of what is happening. Lab-grown cells might allow her to follow what happens over time.
Zhang says he is testing his harm-permitting hypothesis by getting yeast hooked on RNA editing. Baker’s yeast (Saccharomyces cerevisiae) doesn’t have ADAR enzymes. But Zhang engineered a strain of the yeast to carry a human version of the enzyme. The ADAR enzymes make the yeast sick and grow slowly, he says. To speed up the experiment, the strain he is using has a higher-than-normal mutation rate, and may build up G-to-A mutations. But if RNA editing can correct those mutations, the ADAR-carrying yeast may grow better than ones that don’t have the enzyme. And after many generations, the yeast may become addicted to editing, Zhang predicts.
Albertin, Rosenthal and colleagues have developed ways to change the genes of squid with the gene editor CRISPR/Cas9. The team created an albino squid by using CRISPR/Cas9 to knock out, or disable, a gene that produces pigment. The researchers may be able to change editing sites in DNA or in RNA and test their function, Albertin says.
This science is still in its early stages, and the story may lead somewhere unexpected. Still, with cephalopods’ skillful editing, it’s bound to be a good read.