Smell wiring gets set early
Mess with a baby mouse’s olfaction for too long and neurons never recover
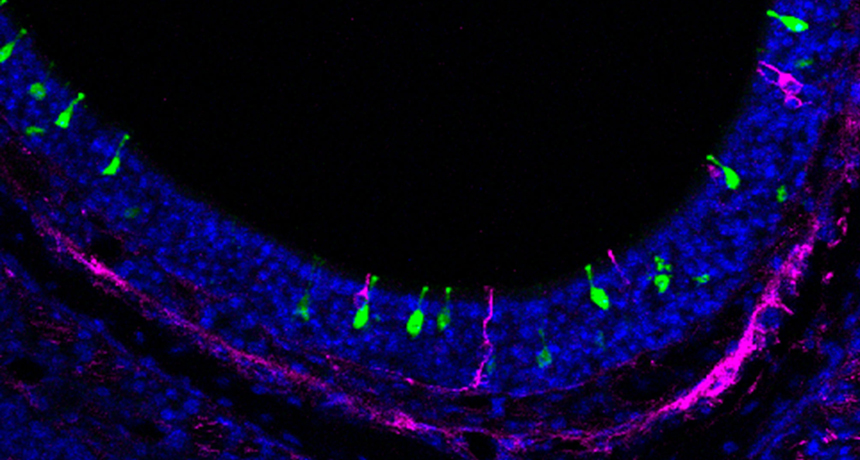
SORTING SMELLS Odor-detecting neurons in the mouse nose (green and magenta) latch onto certain scents and send signals to precise locations. The neurons each have a diameter of approximately 10 micrometers.
Ron Yu and Limei Ma
The intricate neural wiring that carries smells to the brain locks into place soon after birth, two new mouse studies suggest. The results, published in the April 11 Science, identify a window of time in which the olfactory system can be scrambled. Once that window closes, the network becomes cemented into the brain, even as newborn neurons continuously stitch themselves into the mix.
“It is a very important finding,” says neuroscientist Leonardo Belluscio of the National Institutes of Health in Bethesda, Md., who was not involved in the studies. Understanding the details of how the brain’s olfactory machinery builds and maintains itself might lead to insights into how other parts of the brain could be repaired after injury, he suggests.
Some scientists are interested in using neurons produced from stem cells to patch faulty neural wiring, and the new results might one day make that approach more feasible. Figuring out which signals prevent or allow new cells to navigate through the brain might one day help scientists repair brains, says coauthor of one of the studies Gilad Barnea of Brown University in Providence, R.I.
Unlike most places in the brain, the olfactory system is constantly replenished with a steady stream of newborn neurons. To tell the brain what the nose has smelled, these newbies must send message-sending tendrils called axons to very specific blobs of neural tissue called glomeruli. Each neuron carries one type of odor-detecting protein, which determines the axon’s final destination.
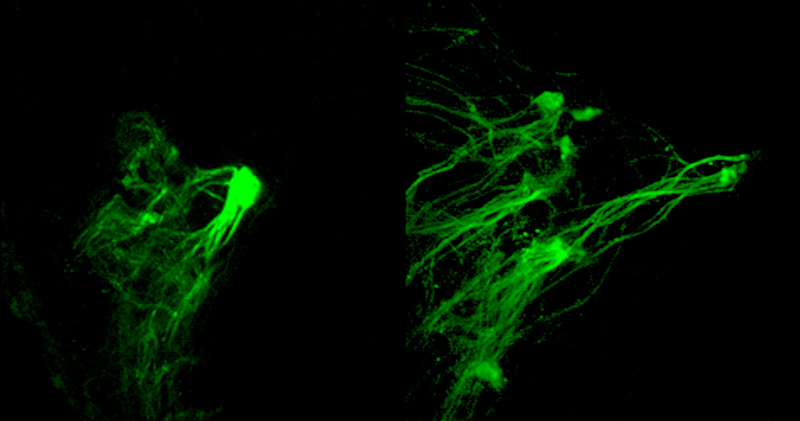
The axons were vulnerable to diversion only for a brief window of time, Barnea and Tsai found. When the engineered odor-sensing molecule was produced right after birth, one mouse of nine laid down faulty wiring. When the molecule was produced starting a week after birth, only one mouse of 17 was affected. Two weeks out, none of nine mice were. The results suggest that the neural wiring is set early in life. “Whatever happens around birth is what takes,” says Barnea.
With the discovery that olfaction wiring can’t be altered after about a week or so of a mouse’s life, smell joins other senses, including vision and hearing, that have critical windows during development.
The second study took the reverse approach: Instead of allowing the wiring to develop normally and then trying to disrupt it, Ron Yu of the Stowers Institute for Medical Research in Kansas City, Mo., and colleagues scrambled the wiring first and then determined if it could recover. A mouse, the researchers found, could regain normal wiring if a disruption was lifted around the first week of life. But after about a week, axons from odor-sensing neurons were less able to reroute to the correct destination. This scrambling left the mice with “interesting changes in their sense of smell,” Yu says, an effect the researchers are still working to understand.
The results hint that as older neurons are replaced by new growth, molecular bread crumbs left behind guide newborn neurons to their correct destinations. “There is some memory in the system,” says Barnea. And this trail of bread crumbs is modifiable, the studies show, but only for a brief window of time early in life.
Although no one knows for sure, Yu says that this memory might be a signal provided by older cells. “The old cells are somehow providing a track that the new ones will follow,” he says. “You simply follow your predecessor.”