Up to Snuff: Nanotube network fights flames
Plastics readily burn. That’s why their makers add fire-suppressing chemicals. But some of these additives have been shown to be harmful to animals and are being phased out. In an upcoming Nature Materials, researchers describe another way to smother plastic’s fiery potential: Include a network of carbon nanotubes.
Brominated fire retardants, which are in everything from computers to furniture, have proved effective, but they accumulate throughout the environment, showing up even in breast milk. Two subclasses of the chemicals have already been banned by the European Union, and, beginning in 2008, they will be illegal in the state of California.
During a fire, plastic melts as the polymer chains within it break down. As the burning progresses, some of the polymer vaporizes, forming gas bubbles that feed and intensify the flames, says Takashi Kashiwagi, a materials engineer at the National Institute of Standards and Technology (NIST) in Gaithersburg, Md. One way to decrease the material’s flammability is to slow production of these flame-feeding bubbles.
To achieve this, some researchers have added a thin layer of nanoscale particles made of alumina silicate to polymer samples. The layer traps bubbles but tends to crack, creating escape routes for some of the bubbles, Kashiwagi notes.
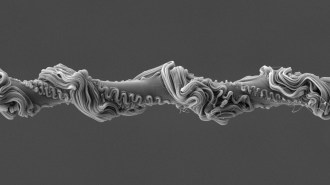
Past work by Kashiwagi indicated that carbon nanotubes—carbon cylinders just a few nanometers in diameter—might be more effective than alumina silicate nanoparticles. For the new experiments, Kashiwagi and Jack F. Douglas, also of NIST, and their colleagues blended various amounts of the nanotubes into polymer samples. “The tubes are like a bunch of stiff coat hangers that you’ve squeezed into the polymer,” says Douglas, a polymer physicist.
When the researchers exposed the tube-riddled polymers to the material’s usual kindling point, bubbles reached the surface more slowly than they did in the tubefree polymers. Plus, the bubbles that did form were smaller. The best tube-filled samples burned only about 40 percent as fast as tubefree samples did.
To suppress flames, the nanotube network had to be dense enough to stem the tide of bubbles. “The network is affecting how the bubbles form and move,” says Douglas.
“They did a beautiful job of investigating the physical effects” of the nanotubes, says Richard E. Lyon, a polymer scientist with the Fire Safety Branch of the Federal Aviation Administration. “The connectedness of the particles is the most important part of this phenomenon, and I don’t think anyone recognized this before.”
Lyon notes that the nanotubes can protect only plastic pieces that are at least a quarter-inch thick, so they wouldn’t work for most pieces found in electronic equipment. But for construction materials, for instance, the new technology “would be very valuable,” he says.