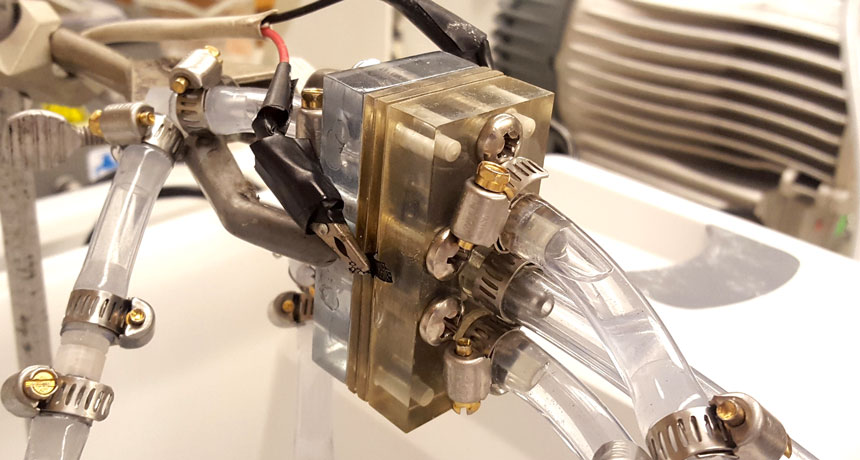
STAYIN’ ALIVE A new type of aluminum-air battery (shown) doesn’t lose its charge as quickly as its kin, thanks to a bit of oil.
B.J. Hopkins
Batteries that use aluminum and oxygen normally live fast and die young. But a new design could help these high-energy devices endure.
Aluminum-air batteries are promising candidates for a new generation of non-rechargeable batteries, because they’re super lightweight and compact. The batteries, however, aren’t widely used because their internal components quickly degrade each other. In the new aluminum-air setup, described in the Nov. 9 Science, oil acts as a buffer between the battery’s corrosive components to greatly extend the device’s shelf life. Such improved single-use batteries could provide backup power to electric cars or supply energy in remote, off-the-grid regions.
“This is a very smart design,” says Yiying Wu, a chemist at Ohio State University not involved in the work. The oil-buffer scheme might also improve other types of metal-air batteries susceptible to self-corrosion, like zinc-air devices, he says (SN: 1/21/17, p. 22).
Each aluminum-air battery cell contains two electrodes, an aluminum anode and a cathode, separated by a liquid called an electrolyte. Oxygen molecules sucked from the air enter the cathode, where they react with electrons and aluminum particles that flow through the electrolyte from the anode, releasing energy to power electronics. Unfortunately, when the battery is on standby, the watery electrolyte eats away at the aluminum anode.
Rethink
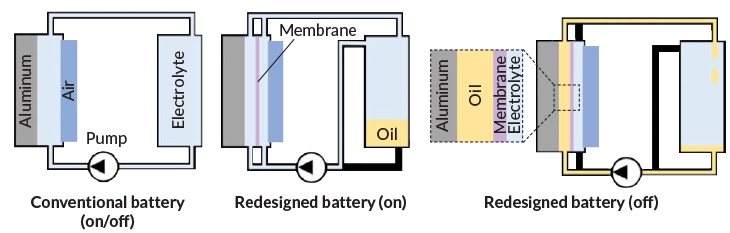
In a conventional aluminum-air battery, the aluminum anode and air cathode are always separated by a liquid electrolyte. In a new version of the battery, an electrolyte is pumped between the electrodes only when the battery is powering another device (middle). When the battery is on standby, oil is pumped into the region between the anode and a polymer membrane to protect the aluminum surface from the corrosive electrolyte (right).
How a revamped aluminum-air battery works
“It’s essentially like a rusty tool,” says study coauthor Brandon Hopkins, a mechanical engineer at the U.S. Naval Research Laboratory in Washington, D.C., who did the work at MIT. “If you put aluminum — or really any metal — in water, it will start to rust and corrode.” As a result, aluminum-air batteries can lose about 80 percent of their stored charge just sitting on a shelf for a month.
Hopkins and colleagues built a more durable aluminum-air device by inserting a polymer membrane between the cell’s electrodes. When the battery is powering a device, electrolyte is pumped into the areas on both sides of the membrane from a reservoir. When the cell isn’t in use, electrolyte is drained from the side of the membrane next to the aluminum anode, and oil flows in to replace it. This oil shields the aluminum from the electrolyte on the other side of the membrane. As soon as the battery is reactivated, the oil is pumped out and stored, and the electrolyte flows back in.
In lab experiments, Hopkins’ team used the new battery cell, as well as an aluminum-air cell without an oil buffer, for five-minute intervals with 24- or 72-hour breaks in between each interval. The electrolyte inside the conventional battery ate through the aluminum anode in just a couple of days, and the cell died. But the electrolyte inside the oil-equipped battery corroded the aluminum anode much more slowly, and the battery worked for a few weeks before the cell’s energy was completely drained.
The single cell prototype’s energy output could possibly be increased either by making a larger version or by stacking multiple cells within a battery pack. “Researchers still need to investigate the performance, cost and reliability of a full-scale battery pack,” Wu says.
In addition to serving as an emergency power source for electric vehicles, such lightweight cells could power long-range drones, Hopkins says. Aluminum-air batteries could also supply off-grid energy to military personnel and civilians in remote areas.






