New analysis rescues quantum wave-particle duality
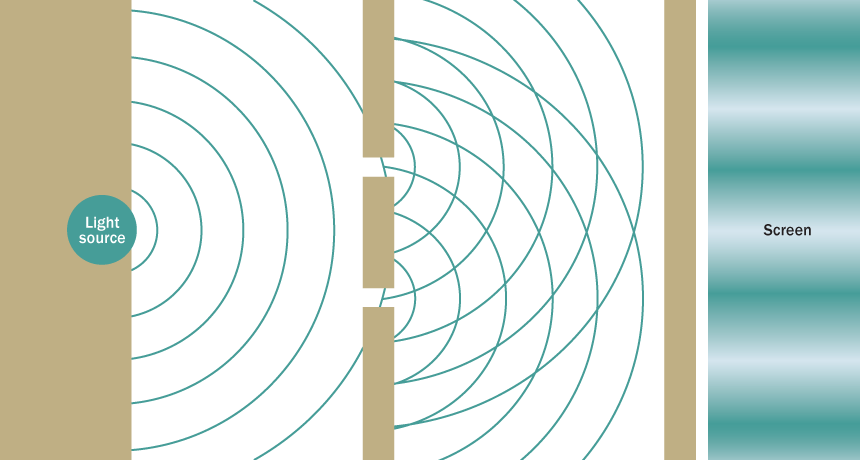
The presence of two slits in a barrier allows light waves passing through to interfere, creating an interference pattern of light and dark bands on a detector screen. Particles, such as electrons, also exhibit such interference and so also behave as waves. But with only one slit in the barrier, both light and electrons behave as particles, producing no interference pattern. This principle of wave-particle duality has been challenged, but a new analysis reaffirms it.
E. Otwell
A basic principle of quantum mechanics has been reaffirmed. Stop the presses. (Or start the tweeting.)
In 2012, experimenters in Germany had supposedly shown that you could observe both wave and particle properties of light in one experiment. That result defied the principle of wave-particle duality: waves can sometimes be particles (and particles can sometimes be waves), but never both at the same time. But now a new paper, published last month in the Proceedings of the National Academy of Sciences, revalidates duality, a pillar in the explanation of quantum mysteries developed by the Danish physicist Niels Bohr in the 1920s.
It’s no surprise, really, for quantum physics to withstand another assault. It would have been shocking if duality had really been overturned. Bohr’s principle requiring the mutual exclusiveness of wave and particle properties in a single experiment had survived many previous challenges. And duality was part of Bohr’s more general principle of complementarity, an idea he developed to address the crises afflicting physics with the rise of quantum mechanics.
When Bohr developed his famous quantum model of the hydrogen atom in 1913, hope was high that the new quantum physics, introduced by Max Planck in 1900, would solve key problems without creating news ones. But Bohr realized otherwise. He knew his atom was a stopgap; atoms more complicated than hydrogen needed an entirely new and more radical recasting of physical theory. And as Bohr and his followers, including Werner Heisenberg, took on the challenge of the atom, other physicists (notably Einstein) worried more about the quantum aspects of radiation. That’s how the wave-particle conundrum crashed the quantum party.
Einstein caused the most trouble. He argued that light traveled through space in the form of particles (later called photons), despite all the evidence to the contrary. Since the early 1800s, most physicists had believed that light consisted of waves, thanks to a famous experiment by Thomas Young. If you shoot light through a barrier with two slits in it onto a surface behind the barrier, you’ll see alternating bands of light and shadow, Young showed. That’s because the waves passing through the different slits interfere with each other, causing brightening in some spots and darkening in others. Had light been made of particles, no such interference would have occurred.
But a century later, Einstein insisted that only photons could explain the photoelectric effect, in which light hitting a metal causes the metal to eject electrons. Eventually Einstein won the Nobel Prize for that paper, although nobody believed him when he published it in 1905.
By the 1920s, though, Einstein didn’t seem so dumb. Experiments on X-rays (basically high-energy light) showed that they carry momentum just like particles do Shortly thereafter, other experiments began to show that electrons, supposedly particles, display properties of waves.
You’d think something as strange as that — particles posing as waves — would have been a shocking experimental surprise, sending theorists scrambling to explain it. But as so often happens in science, the theorists had already figured it out. In this case, the pioneer theorist was Louis de Broglie. He was intrigued by quantum physics and was also a fan of Einstein’s special theory of relativity, which had established the equivalence of mass and energy.
De Broglie had no problem with Einstein’s idea about particles of light. After all, light is electromagnetic radiation, or energy. If energy is equivalent to mass, then it didn’t seem so strange for light to exhibit the properties of particles. But then de Broglie carried that reasoning one step further. If energy (waves) can behave like mass (particles), then why not the other way around?
De Broglie arrived at this insight by realizing the importance of frequency. Early work by Planck and then Einstein had established the key quantum relationship of energy to frequency — energy is simply equal to frequency multiplied by Planck’s constant. In other words, higher-frequency light (or electromagnetic energy more generally) possesses more energy. X-rays, for example, are a high-energy form of radiation with frequency much higher (meaning wavelength much shorter) than visible light.
So if frequency is connected to energy, and energy and mass are the same thing, then masses also should be related to a frequency, de Broglie reasoned. He declared that there must therefore exist “a certain periodic process, of an as yet not more clearly specified nature, which must be assigned to each isolated portion of energy” — that is, particle. And so, he decided, you could assign “to the uniform movement of each material point . . . the propagation of a certain wave, the phase of which propagates in space with a velocity greater than that of light.”
Whoa – faster than light? That would defy Einstein’s relativity, wouldn’t it? Not in this case, de Broglie pointed out, because these waves did not themselves carry any energy. The superposition of these mysterious waves, though, produced another wave that would travel at precisely the same velocity as the particle. So the “traveling energy” carried by the particle could also be viewed as energy being transported by a wave.
De Broglie worked out his idea in 1923 and published his thesis about it in 1924. In 1926, the Austrian physicist Erwin Schrödinger expanded the wave idea to explain the properties of electrons in atoms, in the version of quantum physics known as wave mechanics.
Schrödinger believed the electrons in atoms were simply waves, their orbits consisting of integral numbers of wavelengths. But even before Schrödinger, Heisenberg had worked out an equivalent mathematical description of electrons in atoms, in which the electrons were clearly particles. And despite the new experiments showing wave properties for electrons, all the older evidence that electrons are particles still stood. Same for light, which was still a wave when you wanted it to be, even if it sometimes showed up as particles.
Faced with these issues, Bohr developed his complementarity principle in 1927. He asserted that some mutually exclusive views of nature could both be true, just not at the same time. His prime example was the wave-particle duality. In any given experiment, light (or an electron) could be one or another, but never both.
Bohr illustrated his point with a famous thought experiment analogous to Young’s double-slit demonstration of the wave nature of light. With only one slit in a barrier, electrons would behave like particles, hitting the detector surface at individual points, with no bands of brightness or darkness indicating interference. But with a second slit, the electrons would interfere, producing interference bands. Simple.
But here’s the quantum catch. Even if you sent individual electrons through the barrier one at a time, the presence of the second slit guaranteed an interference pattern — even though each electron could go through only one of the slits. (By the way, it was just a thought experiment in Bohr’s day, but real experiments later confirmed that Bohr was right.)
Bohr’s explanation relied on the fact that even though an electron passed through only one slit, the presence of the second slit meant you (the experimental observer) did not know which slit the electron passed through. If you knew that, you’d be sure it was a particle, and the interference pattern would not materialize. In other words, you could not know which path the electron took (making it a particle) and also observe interference (making it a wave).
That’s just what the 2012 experiment challenged, in a complicated experiment (using photons instead of electrons) in which it seemed you could detect interference and also get information about the photon’s path. But the new paper, by Eliot Bolduc of the University of Ottawa in Canada, with Robert Boyd of Ottawa and the University of Rochester in New York and other collaborators, reanalyzed the challenge and found a flaw.
In real experiments, the relationship between path knowledge and interference is complicated by the presence of the environment. You wouldn’t want to work out the math at home, but the bottom line is that you can have an experiment offering both high probability of predicting the path and high probability of observing the interference. You can choose to measure the part of the environment with the best information about the path, or the part of the environment with the highest visibility of interference fringes. But to test duality, you have to make sure your measurements are equally sensitive to all the possible states of the system (a requirement called “fair sampling”). Bolduc, Boyd and colleagues demonstrated that the 2012 experiment violated the fair sampling rule.
“We show how biased sampling can cause an apparent violation of the duality principle,” they wrote in PNAS. “According to our analysis, the duality principle in its standard form is safe and sound.”
In any case, Bohr’s complementarity principle was never in danger. Wolfgang Schleich, one of the authors of the 2012 paper, points out that it did not claim to violate complementarity, but only “that one is easily misled when thinking naively about this principle.” The new paper, Schleich writes in an e-mail, really does not contradict the earlier observations but simply analyzes them from a different point of view.
In other words, different points of view can both be right, which is exactly what complementarity is all about.
Follow me on Twitter: @tom_siegfried
Editor’s note: Two paragraphs were added to the end of this post on October 6, 2014, to include comments from one of the authors of the 2012 paper.