When the atom went quantum
Bohr's revolutionary atomic theory turns 100

A.B. Lagrelius and Westphal, adapted by S. Egts
Before Niels Bohr, atoms baffled science’s brightest brains.

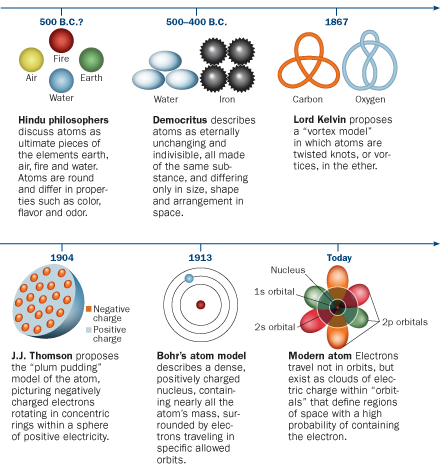
For millennia, atoms had been phantoms, widely suspected to exist but remaining stubbornly invisible — though not indivisible, as their name (Greek for “uncuttable”) originally implied. By the start of the 20th century, physicists knew that atoms had electrically charged parts; the favorite model envisioned blobs of positively charged pudding studded with negatively charged plums (actually, electrons). That image was challenged, though, when Ernest Rutherford showed in 1911 that the positive pudding was all crammed into a massive dense core, or nucleus, surrounded at a distance by the electron plums (SN: 5/7/11, p. 30).
But Rutherford’s atom baffled everyone even more, as the laws of physics prohibited the arrangement that he described. Opposite charges attract each other relentlessly; electrons should spiral into the atom’s positive nucleus in less than a millisecond. (Even if they didn’t, their mutually repulsive negative charges would blast them out of their orbits.) Yet somehow atoms housed negative and positive charges happily.
Into this paradox stepped a great Dane, a genius conditioned by his culture to embrace conflicting ideas and learn from them. A century ago, Niels Bohr married the old standard physics with the new quantum theory, giving birth to the modern model of the atom’s structure.
Bohr’s atom did more than simply reconcile theory with experiment. Bohr figured out the basics of how atoms hook up to make molecules. He explained the mysterious repetition of properties displayed by the periodic table of the chemical elements. And most consequential of all, he established the fundamental role of quantum physics in describing the underlying reality of the universe.
Even though the technical details of Bohr’s model turned out to be wrong, he had grasped the essential idea for understanding atoms: abandoning common sense in favor of the crazy rules of quantum theory. Bohr saw more deeply than others of his time that embracing quantum physics was the key to unlocking nature’s hidden truths. While quantum confusions drove other physicists to despair, Bohr pursued the path into the yellow quantum wood. When two roads diverged, he traveled both but remained one traveler, insisting that knowing reality meant accepting the truth of mutually incompatible viewpoints.
In the decades following his description of the atom, Bohr served as guide and interpreter for the world’s physicists as they explored the strange new quantum world. As the physicist J. Robert Oppenheimer observed, in the development of modern quantum physics, “the deeply creative and subtle and critical spirit of Niels Bohr guided, restrained, deepened, and finally transmuted the enterprise.”
Father of the atom
Bohr’s role in that enterprise began in 1913 with a series of three papers that became the foundation for the future of atomic science.
Bohr “gave the first firm and lasting direction toward an understanding of atomic structure and atomic dynamics,” physicist Abraham Pais wrote in his biography of Bohr, Niels Bohr’s Times (1991). “In that sense he may be considered the father of the atom.”
Like most fathers, Bohr was proud of his offspring. But he was not blind to its faults. He knew from the beginning that his atom model was too simple to capture all of reality’s complexities. He was certain, though, that explaining the atom required quantum physics. “That, of course, was the key to Bohr’s great invention,” says science historian John Heilbron, of the University of California, Berkeley.
Bohr had foreseen the need for quantum theory when investigating the electron theory of metals for his 1911 doctoral dissertation. He found that electrons carrying current and those bound to atoms behaved in different ways, at odds with the ordinary mechanical laws of classical physics.
“He reached the conclusion that there was no possible way classical physics could explain what happened in the behavior of electrons in metals,” says physicist Alfred Goldhaber of Stony Brook University in New York.
Various clues hinted that solving the electron quandary would require Max Planck’s quantum idea, introduced in 1900. From experiments on heat radiation, Planck had deduced that energy could be emitted from a hot object only in indivisible packets called quanta, sort of the way sand consists of individual grains. A few years later Einstein argued that all radiation, including light, was not only emitted but transmitted in such packets (later called photons) even though light was known to travel as a wave.
During the first decade of the 20th century only a few scientists took Planck seriously, and even fewer believed Einstein. But Bohr did. While others deplored the quantum’s contradictions, he exploited them. He had been prepared for the challenge by the circumstances of his upbringing.
Born into an academic family in Copenhagen in 1885, Bohr benefited from a rich intellectual home life. He listened in when the university’s physicist, philosopher or philologist visited his physiologist father for evening discussions. He also absorbed the multiple cultural influences inherent in Denmark’s history and geography, at the crossroads between Germany and England. As children, Niels and his brother Harald listened as their father read aloud from Goethe and from Shakespeare and Dickens. Niels also consumed Danish authors such as Kierkegaard and Hans Christian Andersen and read an unfinished novel by Poul Martin Møller (a mentor to Kierkegaard) called Adventures of a Danish Student. Its discussion of coping with dilemmas and contradictions deeply affected Bohr, impressing him with lessons about language and logic that he referred to throughout his life.
Through his early years of schooling and on to his undergraduate years at the University of Copenhagen, Bohr’s brilliance captivated his professors and classmates. “His family, friends and teachers recognized him as a rare spirit, a thinker at once deep and broad, and helped him in every way to develop his abilities,” says Heilbron.
As he pursued his scientific education, Bohr also learned to appreciate both the German emphasis on theory and math and the British preference for experiment. Destined to be a theorist, Bohr nevertheless chose England for postdoctoral work. He decided to study under J.J. Thomson at the Cavendish Laboratory in Cambridge, the mecca of British experimental physics.
Bohr was eager to absorb the Cambridge magic, both in the lab and in the town. He joined a soccer team and worked on his English by reading The Pickwick Papers, having bought a red dictionary to look up the words he didn’t know.
He was most eager, of course, to talk with Thomson — the electron’s discoverer — about flaws in Thomson’s ideas about electrons in metals. Thomson turned out to be not so interested in hearing Bohr’s criticisms. In late 1911, Bohr met Rutherford, who told him of quantum developments discussed at a recent conference in Brussels. Soon Bohr transferred to the University of Manchester to work with Rutherford’s team, the decisive step toward the quantum atom.
At first, Bohr’s interest at Manchester was still electrons, including the beta particles identified by Rutherford as one form of radioactivity. But Bohr soon realized that radioactivity’s secrets emanated from inside the nucleus. So his search for truth turned to the atom itself.
“Bohr was already on the hunt,” says Goldhaber. “He was looking at every aspect of the atom. And he was going to find out everything that could be possibly found out.”
In the first months of 1912, Bohr worked on the atom problem furiously and fruitfully. In June he wrote to his brother about his progress: “Perhaps I have found out a little about the structure of atoms.” That turned out to be an understatement. In fact, he had determined that quantum physics could make the atom stable.
Bohr wasn’t the first to try to apply quantum physics to atoms. But he showed how to make it work. He pointed out that a proper theory of a stable atom would determine a number with the dimension of length, corresponding to the atom’s size, like the way the length of a spoke determines the size of a bicycle wheel. Producing a number with a plausible length for the atomic spoke was possible only by combining the key quantity in quantum theory, Planck’s constant, with the electric charges and masses of the electron and nucleus.
But explaining how quantum physics governed atomic behavior was not straightforward. In the end, Bohr used classical math for part of his atom model and then mixed quantum physics into it in four specific ways. Two were directly related to Planck’s radiation theory, involving technical aspects of the electrons’ energies. The other two were inspired by processes hidden within the mysterious machinations of Bohr’s enigmatic mind.
One — often celebrated as the crucial ingredient in the Bohr atom model — declared that electrons could occupy only certain specific orbits around the nucleus. In each such allowed orbit the electron possessed an angular momentum equal to a multiple of Planck’s constant divided by 2 pi. With that constraint, Bohr could explain why light was emitted from hydrogen atoms only in certain very specific colors (or frequencies). An emitted color corresponded to an electron jumping from one allowed orbit to another.
Of the many novel aspects of Bohr’s atom, that was the most baffling. Standard physics insisted that the frequency of light should depend on how long it took the electron to orbit the nucleus — its orbital frequency. But if electrons emitted light as they orbited, Bohr pointed out, atoms would radiate light all the time, and they don’t. Hence Bohr demanded that electrons occupy non-radiating orbits while in an atom’s “stationary” state, divorcing the frequency of the light from the frequency of the orbit.
“That cut the ground from under the majority of physicists, who supposed that observable phenomena arising from atomic processes could be linked directly with motion in the microworld,” Heilbron said in April at a meeting of the American Physical Society.
Bohr’s other clever notion offered a way to bridge the gulf between quantum and classical physics. For an electron very far from its nucleus, Bohr said, the frequency of emitted light would be close to the classical prediction. Because distant orbits are very close together, orbital frequencies are nearly equal. So a jump from one to another emits a frequency nearly equal to the orbital frequency. It was another way of saying that for large objects of ordinary experience, quantum effects would be too minute to notice — a key part of the eventual modern understanding of quantum reality.
The atomic constitution
Bohr’s mashup of classical physics with quantum theory offered more insights than would fit in one paper. So he published a series of three, all titled “On the Constitution of Atoms and Molecules,” in the Philosophical Magazine. Part I, appearing in July 1913, described the quantum rules for electron orbits and quantum jumps in the hydrogen atom, explaining the spectrum of colors it emitted. In Part II, Bohr described the arrangement of electrons in rings around the nuclei of more complicated atoms, the first steps toward explaining the periodic table of the elements. Part III described how molecules formed by atoms sharing electrons.
Reaction to Bohr’s theory was mixed. Some experts found it ingenious; others couldn’t understand it. Einstein was intrigued if not convinced at first. But when an experiment confirmed Bohr’s prediction that some colors of light supposedly from hydrogen actually came from helium, Einstein came around. When told of that experiment, Einstein replied, “This is an enormous achievement. The theory of Bohr must be then right.”
But Bohr knew that his theory, while glimpsing a piece of reality, had its deficiencies. Its success, he believed, was largely due to hydrogen’s simplicity. Over the next decade, efforts to apply it to more complicated atoms failed. Finally in 1925 Werner Heisenberg, a young German physicist who had studied at Bohr’s institute for theoretical physics in Copenhagen, constructed a novel mathematical approach that got the right answers. Heisenberg’s paper marked the birth of modern quantum mechanics.
At about the same time, experiments began to show that particles sometimes had wave properties (and vice versa). Erwin Schrödinger constructed a wave version of quantum theory, soon shown to be equivalent to Heisenberg’s particle version. Heisenberg’s work then led in 1927 to his famous uncertainty principle: It was not possible to precisely measure certain pairs of properties, such as a particle’s position and momentum, at the same time.
Once again Bohr stepped in to address the paradoxes. In a 1927 lecture, he proposed a new principle, called complementarity. Light could be particle or wave depending on what experiment you chose to do, Bohr declared. You could measure the position of an electron, or its momentum, depending on how you designed the experiment. You couldn’t do both experiments at once.
Bohr’s complementarity served as the foundation of what came to be called the Copenhagen interpretation of quantum mechanics. In popular discussions, the Copenhagen view emphasizes the role of the observer in creating reality, a point of contention for many physicists today. But Bohr didn’t speak of it in that way, says philosopher of science Don Howard of the University of Notre Dame. It was Heisenberg who focused on the role of observers.
Bohr’s view was much more subtle. He insisted that the properties of a quantum system had no precise meaning before being measured. But measurement required the measuring instrument to interact with the quantum system. Once such an interaction took place, the measuring device and quantum system shared a history — becoming “entangled,” in modern terminology. So how was it then possible to speak of a quantum system’s properties at all?
“Here’s where the really crucial idea entered Bohr’s thinking,” Howard said at the physics meeting. If you specify the experiment you want to perform, you can then use the result to describe a property of a quantum system as if it had a precise value, even if it had no precise value without the measurement. Of course, you couldn’t talk about all the properties of a system at once — you had to choose what to measure.
“For Bohr, two properties like position and momentum are necessary for a complete account of the system and its behavior,” said Howard. “But we could speak of them only one at a time, not simultaneously, because we’re entitled to speak of them as well-defined properties of the system only in a context in which such a property could be measured.” And the measurement contexts for position and momentum are physically incompatible. “That was the deep reason why we couldn’t speak simultaneously of well-defined values of position and well-defined values of momentum,” Howard said.
Multiple truths
Bohr’s embrace of such incongruity reflected views about truth he had developed in his youth. In fact, his investigations of quantum science fed a much broader world view.
“The primary payoff of his engagement with quantum physics for his wider philosophy was the discovery that multiple truths come … in complementary pairs,” Heilbron said.
Bohr’s thoughts on truth have recently been illuminated by newly available correspondence with his fiancée, Margrethe Nørlund, during his work on the atom model. Heilbron cited one letter in which Bohr discusses the different sorts of truths expressed in sermons, great works of literature, and science. The truths of one’s personal sympathies, the universal human truths of literature and scientific truths all differ in kind, but are all important, Bohr wrote. “It’s something I feel very strongly about, I can almost call it my religion, that I think that everything that is of value is true.”
Heilbron sees parallels in these writings to Bohr’s four methods of introducing the quantum into the atom — multiple truths, not all consistent.
“Although they differ in physical content, and sometimes conflict mathematically, Bohr believed that he needed them all,” said Heilbron. “In giving these four formulations, Bohr was not just hedging his bets. He believed that each contained an element of truth and that therefore … he was obliged to use them all even if they conflicted. This principle of inclusion was almost a religious precept to him.”
As for standard religion, though, Bohr was unsympathetic. His mother was a nonpracticing Jew, his father an atheist Lutheran. As a youth, Niels tried to assimilate religious teachings but soon concluded that religion as taught could not withstand scrutiny in the context of logic and science. When he confessed this to his father, the elder Bohr’s response was a simple supportive smile. Niels wrote of that episode to Margrethe: “My courage roared so wildly, wildly, for I knew then that I too could think.”
Heilbron sees in that text a glimpse into the origin of Bohr’s exceptional intellectual journey.
“The approving smile of the man he most admired in the world taught him that he belonged among the few who could reason their way free from standard beliefs of their class and culture, of their time and place,” Heilbron remarked.
And not only could Bohr think, he thought in ways that others could not. He could see that the classical physics enshrined in textbooks “represented the truths of the microworld no better than conventional religious beliefs accorded with the meaning of life,” Heilbron said.
Bohr viewed the aberrations of the quantum world not as heresies to avoid but as clues to deeper truths about reality. His comfort with contradictions enabled him to formulate explanations for quantum paradoxes that have survived the tests of modern experiments, although most of those came after he died, in 1962.
At the time of his death, Bohr was acclaimed as the greatest atomic physicist in the world; he is still widely regarded as the second-greatest physicist of his century, behind only Einstein. Bohr’s legend had developed during the 1920s and 1930s, as beginners from many nations came to Copenhagen to study at his institute. It was there in the mid-1930s that he devised the first clear picture of the internal physics of the atomic nucleus. Soon thereafter, collaborating with the American physicist John Archibald Wheeler, Bohr produced the theoretical explanation for the process of nuclear fission. Bohr’s atom model was then finally fully constructed.
Wheeler once said he wanted to study in Copenhagen because Bohr saw further into the future than other men. How Bohr did that baffled others in much the way that atoms baffled physicists before Bohr. He comprehended nature’s secrets in ways that remain as mysterious as how his weird mixture of quantum and classical physics explained hydrogen’s spectrum.
Perhaps, says Heilbron, the newly released correspondence will offer fodder for new speculations on Bohr’s genius, or even about intellectual creativity in general.
“However these speculations may pan out, they will no doubt bring to light further information linking Bohr’s extraordinary way of thinking, his confident cultivation of ambiguity, his notions of truth and his high culture, to the Danish society that nurtured him,” Heilbron said.
“His like might not be seen again. For as Einstein once said, it’s very remarkable that such a mind as Bohr’s could have existed at all.”
Bohr’s atomic orbits

S. Egts
In Bohr’s model of the hydrogen atom, one electron, carrying a negative electrical charge, circles a nucleus consisting of a single proton, which has a positive charge. Unlike a planet around a star, which could orbit at any distance, an electron can orbit the proton only in certain “allowed” orbits. The size of each allowed orbit is determined by the key numerical quantity of quantum physics, Planck’s constant. An electron jumping from an outer to an inner orbit emits radiation (examples shown) with an energy equal to the difference in the energy levels of the two orbits. When an electron absorbs a certain amount of energy, say from light hitting it, the electron jumps to a higher allowed orbit. Bohr calculated the energy differences between various orbits and found that they corresponded to the observed colors of light known to be emitted by hydrogen.
Follow Tom Siegfried on Twitter at @tom_siegfried.







