Atomic Anatomy
A century ago, Ernest Rutherford inaugurated the nuclear age
Ernest Rutherford grew up in the 19th century. He created the 20th.

No discovery struck deeper into the scientific understanding of reality, with more profound implications for civilization, than Rutherford’s revelation of the architecture of the atom.
A century ago in May, Rutherford published a paper in the Philosophical Magazine interpreting experiments completed two years earlier by his assistants Hans Geiger and Ernest Marsden. They had witnessed the atomic equivalent, as Rutherford later described it, of an artillery shell bouncing backwards off of tissue paper.
It was astounding, unexpected and at first unexplained. Then, after many months of contemplation, Rutherford explained it. The atom had a nucleus.
At the heart of each atom was a single electrically charged kernel, Rutherford surmised, into which almost all of the atom’s mass must be concentrated. The rest of the atom was emptiness, save for the flighty electrons that zipped about at a distance. But Rutherford did not deal with electrons in his 1911 paper. He focused on the atom’s core, its “central charge” — soon to be recognized as a storehouse of energy so vast that Rutherford feared it might be unleashed before the world’s peoples had learned to live in peace.
Rutherford is sometimes regarded as failing to foresee the explosive consequences of his nucleus, having once declared the practical release of atomic energy to be “moonshine.” But he understood better than anyone the immense reserves of energy that the atom had to offer. He was the one, after all, who had figured it out, even before discovering the nucleus.
It was radioactivity that first revealed nature’s hidden source of unimaginable power. Henri Becquerel discovered radioactivity, Marie Curie named it, but it was Rutherford who named its various emissions, revealing that their energies emanated not from chemical exchanges between atoms, but from within the atom itself.
Determined and resourceful, clever and tireless, Rutherford pursued the atom’s secrets with a tenacity that nature had never before encountered. As the greatest experimentalist of his era (perhaps of any era), Rutherford posed question after question about matter at its most basic; nature yielded answers that it had concealed from human knowledge for millennia. By the time he was done, Rutherford had created the atomic age.
He witnessed the prelude to that age as a student at the famous Cavendish Laboratory in Cambridge, England, where he arrived in 1895, an epochal year in physics. In that year a young chemist in New Zealand, J.C. Maclaurin, was named recipient of a scholarship awarded competitively to students within the British Empire for pursuit of graduate studies anywhere they chose. It was an extraordinary opportunity — only one such scholarship was reserved for a New Zealander every other year. But Maclaurin decided to get married instead and turned down the offer. So the scholarship passed to the runner-up, one Ernest Rutherford, son of a farmer and miller in the remote town of Pungarehu. Upon hearing the news, Rutherford exulted that he would never again have to dig potatoes out of the ground. Instead he could begin to dig into the deepest mysteries of atomic physics.
It wasn’t Rutherford’s scholarship that made 1895 famous in physics, though. That year’s signature event occurred in Germany, when Wilhelm Röntgen detected the mysterious radiation that he christened “X-rays.” They immediately caught young Rutherford’s attention. His main physics pre-occupation before traveling to England had been radio waves, so it seemed necessary to investigate whether Röntgen’s rays were similar, or something fundamentally different.
They turned out to be similar in a sense, but much more energetic. With Cavendish director J.J. Thomson, Rutherford established that X-rays passing through a gas generated electrically charged particles. Thomson went on to study cathode ray tubes, showing in 1897 that the beams within them were not like X-rays at all, but rather consisted of minuscule “corpuscles” carrying a negative electric charge. Later known as electrons, Thomson’s corpuscles signaled the demise of the indivisible atom. It was left for Rutherford to figure out how the atom’s parts fit together.
At the time of Thomson’s discovery of the electron, Rutherford had turned his attention to mysterious rays newly discovered to emanate from uranium. This “radio-activity,” he found, was complex; not all the emitted rays were of the same nature. Alpha particles, beta particles and gamma rays emerged as the prime carriers of radioactivity’s energy.
In the midst of these realizations Rutherford left Cambridge for Canada, where he took a professorship at McGill University in Montreal. There he completed the articulation of radioactivity’s physical mechanisms, with special assistance from the chemist Frederick Soddy. Rutherford and Soddy realized that they had identified a novel physical process, inconceivable in a universe with atoms that were indestructible (the long-prevailing view). Previously, atoms had supposedly inviolate identities, having been produced in their present form “at the creation.” All changes in matter had therefore been presumed a result of chemistry — atoms combining and separating with their various types, each forever maintaining its own integrity. But Rutherford and Soddy demonstrated alchemy — atoms changing into atoms of other types. Nothing less could explain the properties that radioactive substances exhibited. Radioactive energy definitely emerged from within the atom — in tremendous quantities, on a scale many thousands of times the range familiar to chemistry.
Furthermore, Rutherford and Soddy remarked in a paper in 1903, this mysterious new form of energy did not appear to be a peculiarity limited to radioactive substances. It was quite clear (to Rutherford and Soddy, at least) that such huge stores of energy were locked up somehow in all atoms. “The energy latent in the atom must be enormous compared with that rendered free in ordinary chemical change,” Rutherford and Soddy wrote. “There is no reason to assume that this enormous store of energy is possessed by the radio-elements alone.” That insight came from experiment, two years before Einstein calculated theoretically the vast amount of energy incorporated in mass.
Return to England
By 1908, Rutherford’s fame was well-established worldwide, and he received the Nobel Prize in chemistry that year (quite an accomplishment, he observed, for a physicist). A year before he had returned to England, assuming the physics professorship at Manchester. Then began the work that introduced the world to the atomic nucleus.
By that time, several scientists had offered guesses at how an atom’s mass and electric charge were distributed. Thomson’s popular “plum pudding” model (an elaboration of a suggestion from the distinguished British physicist Lord Kelvin) pictured electrons (plums) dispersed in a positively charged blob of pudding. Others had shown reason to speculate that negative electrons and positive particles paired up in dyads that occupied an atom with lots of space separating them, like so many mosquitoes trapped in a jar. A Japanese scientist, Hantaro Nagaoka, had proposed an atom that looked similar to the planet Saturn, with a central positively charged planet surrounded by electrons orbiting in a ring. But none of the proposed architectures could claim any experimental justification.
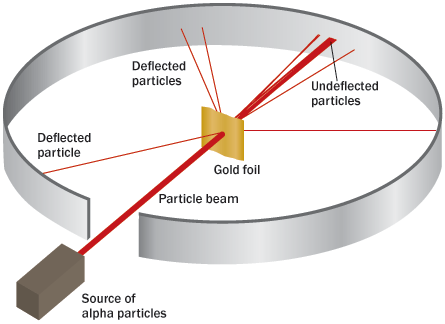
Rutherford advised probing the atom with the alpha particles expelled by certain radioactive atoms. (Alpha particles, Rutherford had correctly deduced, were helium atoms with a positive charge.) Among the targets selected for the alpha probes was a very thin gold foil. A thin enough foil, Rutherford reasoned, would contain few enough atoms that the alpha projectiles should zip through as easily as a bullet through a slab of butter. At Manchester, Hans Geiger began work on the experiments, later to be joined by Ernest Marsden.
One day, Rutherford recounted years later, Geiger excitedly reported that some alpha particles had bounced backward upon encountering the foil.
“It was almost as incredible,” Rutherford recalled, “as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
But it was a couple of years before Rutherford could discern an explanation. No number of small deflections by the gold atoms’ internal electric charges could have diverted an alpha particle’s path so dramatically, he determined, applying some statistical probability calculations (he had once audited statistics classes to prepare himself to deal with such issues). Instead, this type of large deflection could result only from a single encounter, implying that a dense concentration of electric charge sat at an atom’s core.
“Considering the evidence as a whole,” Rutherford wrote in his 1911 paper, “it seems simplest to suppose that the atom contains a central charge distributed through a very small volume.” Soon thereafter Rutherford adopted “nucleus” to refer to the central mass carrying that charge. Calculations showed that such a nucleus extended only about a mere trillionth of a centimeter across, compared with the whole atom’s size of something like a hundred-millionth of a centimeter, 10,000 times greater. In modern imagery, the nucleus was the size of a marble sitting on the 50-yard-line of an atom the size of the entire football stadium, electrons occupying the cheap seats.
At first, no one seemed to notice the dramatic ramifications of the new atom model. Two years later, though, Niels Bohr combined Rutherford’s model with quantum physics to explain mysterious regularities in the light emitted by hydrogen atoms. In the years that followed, the atom-with-nucleus served as the backdrop for the formulation of quantum mechanics, and Bohr much later went on to explain how the nucleus behaved like a drop of (albeit very dense) liquid. Then in 1938, nuclear fission was discovered in Germany; the Manhattan Project soon exploited it to build atomic bombs, and the world entered a nuclear age that redefined the rules of war and peace and exposed the fragility of civilization.
Rutherford didn’t live to see fission or the bomb; he died in 1937, shortly after suffering internal injuries incurred when he fell while cutting a tree branch. It was a loss that other physicists found difficult to fathom. Eulogies recalled his tireless pursuit of experimental answers to every new question; his robust exertion of personal authority with just the right mix of bombast and humility; his penchant for roaming the lab singing “Onward, Christian Soldiers”; his simplicity and directness when dealing with physics and with people. Those who worked with him remembered him forever after as “a rich source of encouragement and fortitude,” Bohr once remarked. “Our memories will always remain irradiated by the enchantment of his personality.”
Follow the neutrons
In the years after the discovery of the nucleus, Rutherford continued to lead the assault on the atom’s secrets, taking over directorship of the Cavendish itself in 1919. He advised or participated in the Nobel Prize–winning work of several other Cavendish scientists, including a landmark experiment by E.T.S. Walton and John Cockcroft in 1932. They split a lithium nucleus into alpha particles, with an energy release providing the first direct experimental confirmation of Einstein’s E=mc2. Rutherford also predicted the existence of a new nuclear particle — the neutron — a dozen years before its discovery at the Cavendish by James Chadwick, also in 1932.
It was the neutron, Rutherford noted, that might permit the atom’s energy to be released on a large scale for practical (such as military) use. During World War I, he had remarked on how a pound of radium contained the energy equivalent of 100 million pounds of coal. He mentioned that he was not eager for anyone to learn how to speed up its release in a world that had not yet learned to live without war.
By the 1930s, Rutherford knew that producing neutrons in copious quantities would enable rapid and vast release of atomic energy. But he still knew of no such source of neutrons, so producing practical amounts of energy from his nucleus did “not look very promising.” Fission’s discovery changed all that, though, as each splitting nucleus released enough neutrons to split others, generating the chain reaction behind both bombs and nuclear electric power generation.
In the year before fission’s discovery, Rutherford’s sudden death at age 66 left physics with a void that remains unfilled. Nobody since has animated the discipline with such a combination of personal presence and scientific power. His old collaborator Soddy remarked that “Rutherford’s death removes from science the most outstanding personality of the age.” Bohr commented that Rutherford “will be missed more, perhaps, than any scientific worker has ever been missed before.” And of the man whom Bohr regarded as “almost like a second father,” Bohr offered a final scientific assessment: “We may say of him, as has been said of Galileo, that he left science in quite a different state from that in which he found it.”