Brain Gain
Constant sprouting of neurons attracts scientists, drugmakers
In the late 1990s, Fred Gage wanted to find a way to see if people, like birds and rodents, continue to produce new brain cells throughout life—a controversial idea at the time. Hearing that Swedish oncologists were injecting cancer patients with a dye that marks rapidly dividing cancer cells, he wondered whether the technique would also highlight newborn neurons in the brain.
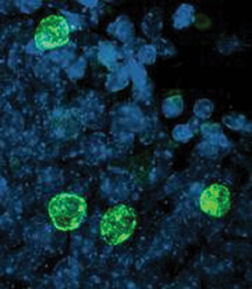

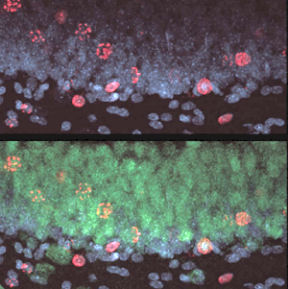
When the patients died, Gage, a neuroscientist at the Salk Institute for Biological Studies in La Jolla, Calif., obtained thin slices of their brains. After examining just five of these samples, he knew he was right: The cancer patients had been making new neurons, a process dubbed neurogenesis.
“This was pretty remarkable, because most of these patients were relatively old, and all of them were very sick,” says Elizabeth Gould, a Princeton University neuroscientist who was the first to report neurogenesis in adult primates. “It was a major turning point.”
Gage’s work helped overturn a decades-old doctrine of neuroscience—that we’re stuck with the brains we’re born with. Instead, it turns out that, like skin, bone, and muscle, the brain—or at least certain structures within it—constantly renews itself.
The research explosion that followed Gage’s discovery generated an expansive list of promoters and inhibitors of neurogenesis. Exercise, estrogen, antidepressants, marijuanalike compounds, stimulating environments, and high social status, along with strokes and other injuries, all rev up production of new brain cells. Aging, stress, sleep deprivation, barren environments, and methylphenidate (Ritalin) damp it down.
As scientists begin to grasp the role of newborn neurons in memory, thought, and regulation of body functions, pharmaceutical companies are leaping toward human trials of drugs that boost neurogenesis. Animal studies suggest that such drugs may provide novel treatments for depression and anxiety, Parkinson’s disease, Alzheimer’s disease, stroke, and even overeating and obesity.
Lost beginnings
Studies of neurogenesis date to the 1960s and 1970s, when scientists at the Massachusetts Institute of Technology (MIT) and Boston University found newborn neurons in certain areas of the brains of adult rats and cats. But in the days before online search engines, this early work faded into obscurity.
“When I was in graduate school in the 1980s, we weren’t taught about adult neurogenesis at all,” says Gould, explaining that researchers had long known that the process existed in youngsters. “I think it was just a classic example of findings and scientists being before their time.”
Renewed attention to adult neurogenesis sprang from two unexpected discoveries. First, Fernando Nottebohm, at Rockefeller University in New York, published sensational reports of fast-growing brain regions in canaries. The more the birds sang, the more neurons they seemed to sprout, Nottebohm and his colleagues detailed in several seminal papers in the early 1980s.
While the work generated widespread interest, most neuroscientists, ignorant of the earlier mammal studies, decided that the phenomenon was a peculiarity of songbirds.
Then Gould, who at the time was working with Bruce McEwen at Rockefeller, revived the idea of neurogenesis in adult mammals. The two were studying the rat hippocampus, a seahorse-shaped part of the brain crucial to memory formation. Removing a rat’s adrenal gland led to massive neuron death in its hippocampus. But in an unexpected twist, the total number of neurons in the hippocampus appeared stable.
Gould and McEwen thought that as some neurons died, others were born.
“It was really an accident in a way,” Gould says of the discovery. “We had this puzzling result that didn’t square with something else we were seeing.” So Gould hit the stacks and dug deep into musty neuroanatomy journals. There, she discovered the MIT and Boston work from earlier decades. Springboarding from those studies, Gould and McEwen reported in 1992 that spikes in stress hormones decreased neurogenesis in rats. Gould then won a professorship in the psychology department at Princeton, launching her career as a pioneering neurogenesis researcher.
Of mice and marmosets
Meanwhile, Nottebohm continued his work in birds. His team captured wild chickadees, injected them with a radioactive dye that marks dividing cells, and released some of them into the wild. The researchers kept the rest captive. After 6 or more weeks, the team recaptured some of the released birds.
The birds that had spent their time flying free had more new neurons in the hippocampus than the birds kept captive did. But Nottebohm didn’t know what factor led to the increase in neuron production.
Follow-up work in Gage’s lab found that mice living in cages with running wheels—which all rodents will spin for hours every night—made more new neurons than did rodents in identical cages without wheels.
Exercise—whether by wing or foot—spurred neurogenesis, it seemed (SN: 2/25/06, p. 122).
Gould then delved deeper into the stress-neurogenesis connection. She and her team set up a colony of marmosets, which are petite primates with funky hairdos. They tested the effects of social stress on neurogenesis by placing marmosets that were unfamiliar with each other in the same cage. Sure enough, the stressed marmosets produced fewer new neurons than did marmosets left on their own. Gage reported similar findings in mice.
In the past 5 years, piles of scientific papers have continued to identify other factors that affect neurogenesis. A bigger picture formed: Nonstressful stimulation and exercise, as well as certain hormones, growth factors, and drugs, speed the birth of neurons, while stress, aging, and sleep deprivation slow the process. Taken together, the recent studies indicate that neurogenesis is “not a genetically preprogrammed process where cells are born and give rise to new neurons at a sort of regular clip,” says Gage. “Instead, there’s this concept that [new neurons] are regulated by experience.”
As interest in the field grew, a more basic question came into focus: What does it mean?
“The most central point in the field is what the cells are good for,” says Hongjun Song, a neurogenesis researcher at Johns Hopkins School of Medicine in Baltimore. “We know that all mammals have the new cells. So why do we need them? If we don’t have them, what’s going to happen?”
The what and the where
It turns out that what new neurons do depends on where they end up. Many arise from a reservoir of brain stem cells near the ventricles, the fluid-filled openings in the middle of the brain. Most then migrate to the olfactory bulb on the underside of the brain, where they presumably help in odor detection.
Some researchers say that they’ve found small stashes of brain stem cells and modest neurogenesis throughout the brain. But those results remain controversial. Only in the ventricles and the hippocampus, especially in a substructure called the dentate gyrus, are researchers confident that brain stem cells regularly spin off new neurons. Given the hippocampus’ key role in memory, scientists zeroed in on it from the beginning.
At first, neurogenesis researchers thought that new neurons might simply replace old, dying ones. “But the view is changing,” says Song, “because it’s almost impossible for the young cells to make exactly the same connections as the cells [that die]. What we’ve found, and what other people have found, is that the young cells seem to be different than the old cells. . . . The young cells are very plastic, they’re very flexible.”
Like babies exploring the world, young neurons in the dentate gyrus spend their first weeks absorbing information that programs them for the rest of their existence, according to a theory Gage and others are developing. If the nascent cells are starved of stimulation, they wither and die. “It seems that something is coded in those [new] cells during that critical period,” says Gage. He says he thinks that this ability makes new information more prominent. Consensus is building that “the new neurons are doing something important for learning,” says Gould. In rodents, for instance, neurogenesis appears crucial for spatial memory, needed in tasks such as remembering how to navigate a maze.
Harnessing new neurons
Even as researchers uncover how the brain generates new cells and how these cells knit themselves into the brain’s networks, companies are already testing compounds for their ability to stimulate neurogenesis.
“If we have new neurons, can we use them to remake [brain] circuitry and to cure disease?” asks Song.
Drugmakers’ interest grew in 2000, when a team at Yale University reported that antidepressants such as fluoxetine (Prozac) increased neurogenesis in the hippocampi of rats. The finding helped explain how the drugs work. While antidepressants boost neurotransmitter levels immediately, patients don’t feel better for several weeks, which puzzled psychiatrists. But because new neurons don’t mature in the hippocampus until several weeks after the start of drug therapy, the Yale team speculated that they may have stumbled on the real way antidepressants work.
A series of follow-up experiments by René Hen and colleagues at Columbia University buttressed the theory. The team treated mice with antidepressants but inhibited neurogenesis by zapping the animals’ brains with X rays. Minus neurogenesis, the animals didn’t respond to the drugs.
“[T]hat’s what got us convinced that neurogenesis had something to do with the mechanism of action of antidepressants,” says Hen.
Recent work in monkeys lends further support to the idea. In a paper published in the May 2 Journal of Neuroscience, a team led by Tarique Perera of Columbia University found that electroconvulsive shock therapy, a last-resort treatment for depression in people, boosts neurogenesis in adult monkeys. The study is the first to show that depression treatment increases neurogenesis in primates.
Researchers are now exploring whether boosting neurogenesis in brain areas other than the hippocampus might benefit patients with Alzheimer’s, Parkinson’s, and other diseases. “There is increasing evidence that there are dormant [brain stem cells] in different parts of the brain, and with proper stimuli these quiescent cells can be woken up [to] start producing neurons,” Hen says. Whether this will help patients remains unknown.
A Swedish company, NeuroNova, plans to test the idea in people late this year. Using an undisclosed growth factor, the company hopes to induce neurogenesis and other brain changes to restore function in patients with Parkinson’s disease. The growth factor will be delivered via a tube directly into the brains of 10 to 20 patients with advanced Parkinson’s, says Anders Haegerstrand, NeuroNova’s chief scientific officer. “We’ve done experiments [in animals] where we see an increase in dopamine cells”—a type of neuron—”in the substantia nigra,” he says, referring to the brain structure that degenerates in Parkinson’s patients.
But there’s a danger. Stimulating too much neurogenesis willy-nilly can cause seizures, says Phyllis Wise, who studies animal models of stroke at the University of Washington in Seattle. In animal models of epilepsy, seizures induce rapid growth of new neurons, which in turn seem to feed further seizures. “Not all neurogenesis is necessarily good,” says Wise. “It has to be controlled.”
Another company, Brain Cells Inc., founded by Gage, Hen, and other scientists, is planning a clinical trial of a new antidepressant later this year. The company’s scientists discovered the compound with a test that screens for neurogenesis. “Many, many big pharmaceutical companies, as well as small start-ups, have identified this [test] as something they can use” to find new brain drugs, says Gage.
In addition to depression and Parkinson’s disease, animal research suggests that enhancing neurogenesis may have some value in treating Alzheimer’s disease and even obesity. One mouse study published in 2005 found that treating fat rodents with a brain-growth factor spawned new neurons in the hypothalamus, which serves, among other functions, as an appetite regulator. Treated animals lost substantial weight.
The trouble with human studies, according to Hen, is that no one knows exactly what brain changes contribute to observed outcomes. Neurogenesis may be just one process among many that allow the brain to adapt. Antidepressants may indeed drive neurogenesis, but a host of other brain changes, such as denser connections among neurons, could also help explain how the drugs work. In her experiments with marmosets, Gould keenly monitors this synaptogenesis as well as neurogenesis, because she’s interested in how social interactions change the brain as a whole. And because human brains can’t be readily sliced and stained to reveal new neurons, “we really need some good imaging techniques,” Hen says.
A paper published in the March 27 Proceedings of the National Academy of Sciences brings that wish a step closer. The study, led by Scott Small of Columbia University, found that, in mice that exercised, increased blood flow in the brain indicated neurogenesis. Using magnetic resonance imaging scans, the team then saw the same increased hippocampus blood flow in people who exercised. “We’re relying on inference to say that what we’re seeing probably reflects neurogenesis in humans, but there’s no way to know with certainty,” Small says.
Says Gould: “A good imaging technique would do wonders for the whole field.”
In the meantime, Gould and her colleagues are basking in the glow that comes from overturning scientific dogma. “When we had our first abstract at the Society of Neuroscience meeting in the early 1990s, there was no session for us,” says Gould. “Now I see session after session of just adult neurogenesis. It’s really amazing. There’s a lot of data pouring in.”