In the summer of 2003, geologist Leslie A. Melim and two of her undergrads were exploring a 6-meter-by-12-m chamber deep within New Mexico’s Carlsbad Caverns. As the team members were on their hands and knees conducting a detailed survey of the cave floor, one of the students blurted out, “Hey, what’s this?”

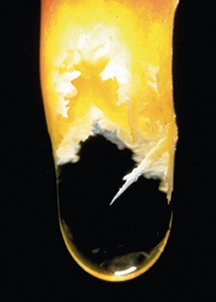

“This,” says Melim, was an oddly configured carpet of white minerals lining a shallow basin that had long ago, when the region’s climate was wetter, held water. The sharp, wispy peaks of the carpet were less than 1 centimeter tall and 1.5 cm across at their bases, resembling crests atop a lemon meringue pie.
“It was unlike any cave formation I’d seen or heard of, and I was pretty sure no one else had seen it before either,” says Melim. Considering the location of the find and its appearance, the team from Western Illinois University in Macomb dubbed the mineral coating “pool meringue.” The group has since found similar formations in two other dried pools in Carlsbad’s lower reaches.
As at Carlsbad Caverns, many treasure vaults lie deep beneath Earth’s surface in cathedral-like chambers, accessible only through narrow passages, often with fanciful names such as Contortionist’s Delight or Fat Man’s Squeeze. In these pitch-black and usually humid confines, mineral formations range from iciclelike deposits that can weigh tons to delicate crystals that shatter at the slightest touch. Although scientists have long understood the chemical processes that sculpt many cave formations, they’ve only recently come up with a mathematical model that explains some of their shapes.
An even fuller explanation of the intricate decor will also have a biological component, according to researchers who are characterizing the unusual bacteria that live in caves. In Mexico’s Cueva de Villa Luz, as in many others, moist surfaces bear gleaming coats of mineral-depositing bacteria, filmy ribbons of their microbial kin undulate in cave-floor pools and streams, and damp globs of microorganisms hang from the walls. Some types of mineral deposits that form in caves appear to be produced by communities of bacteria or by the environmental conditions that they create. On the opposite side of the coin, some of the same organisms are threatening prehistoric cave art.
Dangling depositsCave formations come in numerous shapes and sizes, and many of their names—popcorn, cave bacon, and soda straw, for example—seem to have sprung from the minds of imaginative—and hungry—cavers. The two formations best known to noncavers, however, bear the names stalactite and stalagmite. Pity the grade school test taker who forgets that the c in stalactite stands for “ceiling” and the g in stalagmite stands for “ground.”
Stalactites, stalagmites, and other cave deposits form when water picks up minerals as it percolates through sediments and then seeps into a cave. If the water has traveled through limestone on its journey, it typically is saturated with calcium carbonate and carbon dioxide, says Raymond E. Goldstein, a physicist at the University of Arizona in Tucson. This seepage can also contain trace amounts of a wide variety of elements.
In the first fraction of a second after water seeps from a cavern ceiling, some of the gas dissolved in the seepage enters the humid air because the cave’s air isn’t saturated with carbon dioxide. This process, a gentle fizzing like that in soda pop, causes the water droplet to become less acidic, Goldstein says. As a result, some of the calcium carbonate in the droplet crystallizes on the cave ceiling, and—voilà!—a stalactite is born.
As seepage continues, new droplets hang from this bump and leave their crystalline residue. When droplets fall to the cavern floor, the dissolved minerals that they carry often accumulate there as stalagmites. When a stalactite and its underlying stalagmite have nearly grown together, the deposits’ shapes are almost perfect mirror images. Further growth melds the formations into a column with a narrow waist, which continues to thicken as the minerals precipitate from the water flowing over the surface.
The growth of a stalactite is incredibly slow—typically, it takes a century to add a centimeter of length. At first, stalactites grow irregularly, but once they’ve reached a length of 5 cm or so they take on a characteristic shape. From the side, the formation doesn’t look like a perfect cone but bulges like a plump carrot, says Goldstein.
Although scientists had long recognized this distinct profile, no one had explained how stalactites end up with their bulging shape, he notes. Now, Goldstein and his colleagues have come up with a mathematical model that reproduces a stalactite’s silhouette.
Earlier experiments had shown that the rate of mineral precipitation on each small patch of stalactite correlates with the thickness of the layer of water that’s dribbling down the formation. At the top of a stalactite, where the layer of flowing water may be only a few micrometers (µm) thick, calcium carbonate is deposited slowly, says Goldstein. Near the bottom of the formation, where the flowing water is spread over a smaller area, the film of water is thicker and crystals form more quickly.
Because the top of the stalactite has a larger diameter and more surface area than the bottom does, more rock is deposited there overall. However, the more-rapid mineral deposition at the bottom portions widens the formation to create the characteristic carrot profile. Goldstein and his colleagues reported their findings in the August 2005 Physics of Fluids.
When the seepage rate into a cave is extremely slow, mineral-laden droplets can hang on the ceiling for a long time before they fall. In such a case, the calcium carbonate crystallizes in a ring shape. The crystals that precipitate from subsequent droplets extend the ring into a delicate tube, generating a so-called soda straw that can grow several meters long, says Goldstein.
Only about 1 percent of the calcium carbonate that’s dissolved in the water flowing down a stalactite remains on that formation, Goldstein and his colleagues estimate. The rest is carried to the cave floor within the dripping water. Over time, the deposits from drips can produce stalagmites.
Although his team’s model doesn’t address those structures’ formation, Goldstein speculates on how a stalagmite’s shape develops. As stalactites do, stalagmites grow irregularly at first. The crystals accumulate in a broad, random pattern, probably because the mineral-rich droplets splash when they hit the cave floor, says Goldstein. As the mineral layers thicken and the formation grows upward, the distance through which the droplets fall becomes shorter, so the droplets splash less when they strike and the stalagmite takes on a more predictable carrot shape that’s often the mirror image of the stalactite above it, he proposes.
Built by bugs?
Hundreds of types of rock appear in cave formations. The most abundant ones are forms of calcium carbonate deposited when mineral-rich waters seep into an open space underground. Increasingly, however, researchers are finding that many exotic cave formations are in one way or another associated with bacteria.
For instance, some of the walls in New Mexico’s Lechuguilla Cave are coated with gnarly bumps of various sizes, a type of formation that cavers have nicknamed popcorn. The smallest bumps are 2 to 3 millimeters across and look more like bacterial colonies growing in a petri dish than like popcorn, says Hazel A. Barton, a microbiologist at Northern Kentucky University in Highland Heights. The largest knobs of the hard, white rock are thumb-size replicas of the popped kernels of the snack that shares their name.
Microscopic analyses of the bumps and knobs show layers of bacteria that have been fossilized in calcium carbonate. Barton and her colleagues have scraped samples of unidentified live bacteria from cave walls and took them back to the lab. When fed a calcium-containing substance, the microbes made crystals of calcium carbonate, the same material that encases their fossilized brethren.
Microbes are present on most moist cave surfaces. However, because not all such bacteria can be cultured in the lab, it’s often difficult to confirm which organisms, if any, are responsible for forming cave minerals, says Brian Jones, a geologist at the University of Alberta in Edmonton who has studied cave microbes.
In some instances, bacteria appear to play a more indirect role, merely creating the environmental conditions under which dissolved minerals are more likely to crystallize. For example, quartz pebbles and rocks in many cave-floor streams are coated with a layer of manganese oxide–containing minerals that can range from fractions of a millimeter to a few millimeters thick, says William B. White, a geochemist at the Pennsylvania State University in University Park. The predominant mineral in the coatings is birnessite, a manganese oxide compound that includes traces of sodium, calcium, and potassium.
Microscopic analyses of the birnessite coatings reveal many fossilized bacteria, so White speculates that those organisms derived energy from the manganese dissolved in the water that flowed over them. During the energy exchange, the manganese ions were converted to an insoluble form that then precipitated to form the birnessite.
At this stage of research, White says, it’s unclear whether the microbes had a role in the coatings’ formation or whether they simply inhabited the veneer after it formed.
The pool-meringue formation in Carlsbad Caverns may represent a stronger link between microbes and cave minerals. Scattered throughout the meringue are smooth tubes 2 to 3 µm long and less than 1 µm in diameter. That’s the right size and shape to be microbial filaments, says Melim.
Lab results also hint that the filaments have a microbial origin. Compared with the rock that encased them, the microscale tubes are more resistant to mild acid and contain slightly more carbon.
Starvation diet
Ecologically, caves are some of the world’s most isolated environments. That seclusion offers stability: In large caves, temperatures rarely fluctuate, and the humidity in caves receiving a steady flow of mineral-rich groundwater stays close to 100 percent. “It’s like a sauna, only a lot cooler,” says Jones.
The isolation and stability also make several cave features valuable recorders of geologic and even climatic history. The ratio of oxygen isotopes in a sample of carbonate can provide clues about the temperature at which the cave mineral crystallized. While soil temperatures at or near Earth’s surface rise and fall with the seasons, these fluctuations are tempered in deep caverns. Therefore, a deep cave’s temperature matches the average annual temperature at ground level directly above it.
Although useful to scientists, a cave’s isolation from Earth’s surface causes problems for organisms living there, says Barton. It’s pitch-black deep inside a cave, so no food chain can be based on photosynthesis. Many microbes obtain energy by breaking down rocks and taking advantage of the chemical energy from those reactions (SN: 11/15/03, p. 315: Attack of the Rock-Eating Microbes!).
Most of the nutrients available to support life are those carried in by groundwater. Each liter of water seeping into a cave typically carries between 15 and 50 micrograms of organic carbon, about one-thousandth the concentration that’s considered a starvation diet for microbes living at Earth’s surface. Forced to make a living from such slim pickings, the cave-dwelling organisms have developed unusual techniques for extracting energy from their surroundings.
“These bacteria are incredibly starved, but they’re incredibly diverse,” says Barton.
With that variety in the face of adversity, microbes have evolved complex communities that work together to efficiently process the minuscule quantities of nutrients that flow their way, she notes. These communities take many forms, including slick films on rock walls, mats in the cave’s streams and pools, and moist globs that geologists have dubbed snottites.
Microbes quickly take advantage of any nutrients brought into the cave by human or animal explorers as well as by seeping water, says Penelope J. Boston, a microbiologist at the New Mexico Institute of Mining and Technology in Socorro. Ropes installed by spelunkers in Lechuguilla Cave are being slowly consumed by fungi, she notes. Tubing used to siphon drinking water from cave pools, if left dangling into the water, soon gains a coating of microbes that derive nutrition from organic compounds that leach from the plastic. Even the hair and skin cells shed by the occasional caver provide a source of nutrition for cave bacteria, says Boston.
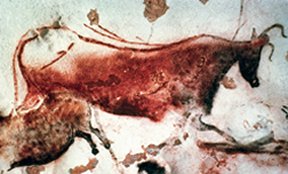
The microbial penchant for consuming any substance with nutritive value poses a threat to some of humanity’s priceless works of art, the cave paintings that are scattered across much of Europe. Some of those works date back to the midst of the last ice age. To make their paints, the prehistoric artists mixed minerals such as iron oxide and organic substances such as charcoal into binders such as vegetable oils and fats—appetizing ingredients all, for a starving microbe.
Recent surveys have found a wide variety of bacteria living on or near cave paintings at several sites, says Cesareo Saiz-Jimenez, a microbiologist at the Institute of Natural Resources and Agrobiology in Seville, Spain. Some of the more-renowned masterpieces under attack include cave art in France’s Lascaux Cave and Spain’s Altamira, La Garma, and Tito Bustillo caves. Scientists haven’t worked out lab methods to grow many of the microbes attacking the paintings, so those organisms haven’t been studied in detail. However, analyses of their DNA provide hints about their family relationships and lifestyles.
For example, cave paintings in Altamira Cave support microbes from the group Crenarchaeota, which can be found in many soils but also inhabit extreme environments such as near-boiling springs, Saiz-Jimenez and his colleagues reported in the January Naturwissenschaften. The same paintings also host microbes from the genus Acidobacteria, which often thrive in acidic soils. Such microorganisms threaten not only the cave art’s pigments but also the underlying rock.
Attempts to kill or control the art-loving microbes with disinfectants might not work, say some cave-art conservators. They’re concerned about damage by such cleansers to the rock. Furthermore, killing some of the microbes might simply shift the balance of power to an even more destructive set of organisms and thus accelerate damage rather than prevent it.
The best hope for the cave art, says Saiz-Jimenez, lies in continued research, which may yield insights into how to inhibit the growth of microbial communities and how to minimize the damage they’re causing.