Channel Surfing
Atomic-resolution snapshots illuminate cellular pores that control ion flow
Sarah Hughes unexpectedly skates to a gold medal in Salt Lake City at the Winter Olympics. Barry Bonds whips a baseball bat around to smack yet another home run. At the age of 22, Garry Kasparov defeats Anatoly Karpov to become the youngest world chess champion ever. Physicist Stephen Hawking teases out the mysteries of a black hole. Those are celebrations of human physical and mental capabilities.
A man writhing in an epileptic fit isn’t. Nor is a child with cystic fibrosis struggling to draw air into clogged lungs, or a promising young basketball player who dies suddenly of heart failure.
Microscopic cellular pores called ion channels unite the starkly different images above. These protein-based molecular gatekeepers govern the cellular influx and outflow of ions, such as calcium and chloride. Literally every single thought or action involves these channels. After all, among their duties is regulation of the electrical excitability that nerve cells use to communicate and that muscles exploit to contract.
“There’s no question that nature uses ion channels to accomplish many things in the cell. They’re obviously very important for life and health,” says Roderick MacKinnon, a Howard Hughes Medical Institute (HHMI) investigator at Rockefeller University in New York.
When an ion channel malfunctions, the outcome can be devastating. Epilepsy can result from defects in channels for calcium, while cystic fibrosis stems from mutations in a channel for chloride ions (SN: 1/26/02, p. 59: Available to subscribers at The Persistent Problem of Cystic Fibrosis.).
Problems with potassium channels have been known to cause arrhythmic beating even in an athlete’s well-conditioned heart.
Those are just some of the reasons why scientists want to better understand how ion channels work. Moreover, many drugs, including ones for epilepsy, heart conditions, and migraines, work by altering the function of such pores. Uncovering their molecular structures should help researchers design new agents that work by binding to the channels.
Yet until recently, scientists were hamstrung in their efforts to depict channels’ structures. In 1998, however, MacKinnon and his colleagues used X rays to construct atomic-resolution images of a potassium channel. The detail was unprecedented. These snapshots provided immediate insight into questions such as how the channel can shuttle up to 100 million potassium ions across a cell membrane in a single second while keeping out similarly charged sodium ions, whose smaller size would seem to make passage even easier.
The revelation of the long-awaited potassium channel’s structure drew strong praise. “A dream come true for biophysicists,” said Clay Armstrong of the University of Pennsylvania School of Medicine in Philadelphia in a commentary accompanying the 1998 report.
MacKinnon and other researchers have continued to delve into the structure and function of the potassium channel. And earlier this year, MacKinnon’s team published the first atomic-scale structures of a chloride channel. As with the earlier work on the potassium channel, the new images have helped explain how the complicated chloride channel operates.
“The drug companies are just drooling at these structures,” notes Stephen Cannon of Massachusetts General Hospital in Boston.
Powerful crystals
Discovered in the 1950s, ion channels have been the topic of thousands of studies. In one common research strategy, investigators mutate a channel’s gene to exchange or eliminate a single amino acid in the protein. If this changes the function of the channel, researchers have a new clue to its structure. This technique might reveal, for example, that the amino acid glutamate in one specific location of the channel protein serves as part of the gateway for ions.
Such studies have provided some insight into how channels work, but they haven’t delivered the clarity that comes from, say, a picture of a channel’s three-dimensional shape. It’s akin to the differences between inferring a large object’s shape through touch versus seeing it all at once with your eyes.
For years, however, many researchers didn’t think that direct images of ion channels were possible. To get atomic-resolution images of a protein, scientists generally turn to X-ray crystallography. They first need to coerce many billions of the protein molecules to crystallize, and then they shine X-rays on the resulting crystal. From the way the X-rays bounce off the protein, the investigators can painstakingly deduce the location of each atom and ultimately reconstruct the protein’s shape.
That’s the ideal, anyway. The practice isn’t easy when it comes to ion channels because they snake in and out of a cell’s membrane, making them difficult to isolate. Investigators must first use a detergent to extract the channels from cell membranes. But the detergent itself sticks to the protein and impedes its forming a well-ordered crystal.
Another major challenge is to obtain enough copies of an ion channel protein to produce a crystal. Individual cells have relatively few ion channels of any particular type. That’s why MacKinnon and other scientists usually resort to studying the ion channels of bacteria. These structures closely resemble ion channels in more complex creatures, and it’s easy to culture the microbes in large batches.
In 1998, using this approach, MacKinnon and his colleagues produced the first detailed structure of an ion channel–a potassium channel called KcsA from the bacterium Streptomyces lividans. In animals, potassium channels govern nerve cell signaling, beating of the heart, and release of insulin.
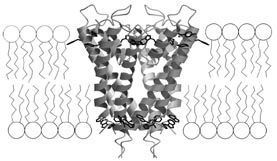
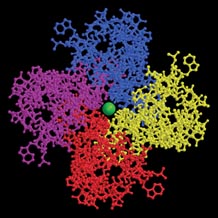
Scientists have known for years that potassium channels are composed of four identical protein subunits that form a complex. The crystallography-derived images of KcsA confirmed that and showed that the channel is an inverted teepee with the four subunits providing the supporting poles.
The pictures also immediately cleared up some mysteries surrounding potassium channels, such as how the channels distinguish so well between potassium and sodium ions. A potassium channel lets in just one sodium ion for every 1,000 or so potassium ions, although each sodium ion has the same electrical charge as a potassium ion and are only slightly smaller.
It turns out that the wide exterior opening of a KcsA channel contains what scientists call a selectivity filter. Potassium ions, stripped of the water molecules that normally surround them when dissolved in body fluids, just fit inside this oxygen-lined tunnel structure. The filter’s oxygen atoms essentially take the place of the water molecules.
“The potassium channel is organized to be a mimic of water,” says MacKinnon. “The design is very simple and very beautiful.”
Sodium ions are too small for a potassium channel’s oxygen lining to effectively take the place of the ion’s shell of water. As a result, the sodium ions generally remain outside the channel.
The images of the KcsA channel “explained beautifully why sodium can’t go through the channel,” says Bruce Bean of Harvard Medical School in Boston.
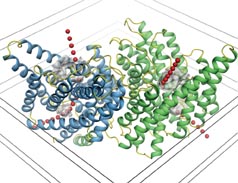
The new structural data also offered details on how the channel speeds potassium ions into the cell. Various stretches of amino acids within the channel coil up into helical structures whose distributed electrical charges draw positively charged ions from the opening of the channel toward the inside of the cell. There’s also a minuscule water-filled cavity within the pore that the potassium ions enter after flowing through the selectivity filter.
Reacquiring a shell of water there may help an ion complete its passage through a cell’s oily membrane into the watery interior, suggest researchers.
Since its 1998 publication, MacKinnon’s team has teased more and more information from their crystallography-derived images. In the June 7, 2001 Nature, for example, the investigators described a portion of the channel protein that can plug up its pore very quickly and shut off the flow of ions. The time it takes for such a channel to reopen–on the order of a millisecond–explains in part why nerve cells need tiny breaks when they fire off electrical signals, notes MacKinnon.
In the Nov. 1, 2001 Nature, the researchers presented the highest-resolution images yet of the bacterial potassium channel. Among other things, the pictures reveal that as many as four potassium ions at a time can flow through the pore across the cell membrane.
Negative thoughts
Before folding into their three-dimensional shapes, proteins start off as linear strings of amino acids. Because of similarities in the amino acid sequences of the proteins that comprise channels for positively charged ions, scientists suspect that sodium and calcium channels will prove to have the same basic structure as the KcsA channel.
Until a few months ago, it was less clear how the channels that conduct negative ions into and out of cells do their job. In the Jan. 17 Nature, MacKinnon’s team hits pay dirt again, this time presenting the structures of bacterial chloride channels.
Before this work, scientists weren’t even sure which parts of a chloride-channel protein assembled into the pore structures. With pictures of these channels in hand, it’s now understandable why investigators struggled so much.
The chloride channel is “such a complex structure, one in which the pore involves many segments of the channel,” explains David Clapham, an HHMI investigator at Children’s Hospital in Boston.
The new work resolves a lingering controversy over whether the channels harbor one pore or two, one in each subunit. MacKinnon’s pictures show “unequivocally” that a chloride channel has two pores, says Cannon.
The chloride-channel structure is markedly different from that of the KcsA channel. Instead of inverted teepees, the chloride channels resemble hourglasses. Ions flow into a large opening that narrows considerably at the middle of the channel before expanding again.
In some ways, however, the chloride and potassium channels are similar. Both channels contain helical structures that have a distributed electrical charge.
In contrast to the helices in KcsA channels, however, those in the chloride channels are oriented so that the positively charged faces of the helices point inward. This helps draw the negatively charged chloride ions into the channel and through to the cell’s interior. “It’s the same thing [as in KcsA channels] but in reverse,” says MacKinnon.
Head to toe
Determining how channels selectively permit certain ions to enter a cell is just one of two crucial issues concerning the pores. The other centers on how channels open and close like a valve, a process called gating. Some channels open when they receive a chemical or environmental signal, such as the recently discovered calcium channel that responds to cold temperatures (SN: 2/16/02, p. 101: Available to subscribers at Cool Discovery: Menthol triggers cold-sensing protein.). Other channels open in response to changes in the voltage differential across the surrounding cell membrane. Because they can open and close so quickly, these so-called voltage-gated ion channels are well-suited for their roles in nerve conduction
Revealing the mechanisms behind voltage gating is, in the words of Cannon, “the next Holy Grail” in the ion-channel field.
Neither MacKinnon nor anyone else has yet produced atomic-resolution pictures of a voltage-gated ion channel. One difficulty has been the inability to find a suitable channel for crystallization. It appears that Clapham’s team may have taken a major step in that quest, however.
The researchers recently discovered a novel voltage-gated calcium channel in the tails of mammalian sperm (SN: 10/13/01, p. 228: Sperm Protein May Lead to Male Pill). They then looked at various databases of genetic sequences in search of similar channels in other species. They found a promising candidate in a bacterium called Bacillus halodurans, which lives in high-salt environments. However, the microbial channel conducts sodium, not calcium.
“People had predicted this channel would be a calcium channel . . . so it was a big surprise that it turned out to be a very fast-activating sodium channel,” says Clapham.
There was something even more surprising. No one had previously reported voltage-gated ion channels in a microbe. Jellyfish were the simplest creatures known to possess such channels. It was generally thought that microbes, which lack muscles and nervous systems, don’t need the high-speed reactions that voltage-gated ion channels permit.
“This changes the whole evolutionary picture of [ion] channels,” says Clapham. “It means that bacteria, the most primitive life forms, have what was thought to be a very specialized channel.”
Beyond raising questions about the origins of various ion channels, the protein identified by Clapham’s group may also finally allow researchers to obtain an atomic-scale portrait of a voltage-gated channel. Clapham and several other teams are now racing to grow B. halodurans and obtain enough of its channel proteins for crystallization.
“This [sodium channel] is going to be the tool that cracks the mystery of voltage sensing,” predicts Cannon.
If that turns out to be true, scientists will have answered the most fundamental questions about ion channels. While he’s optimistic that the currently known structures of ion channels, as well as ones that will be uncovered in the coming years, will ultimately lead to novel drugs, MacKinnon admits that he’s motivated more by the thrill of understanding these remarkable proteins.
“I just wonder how nature does things,” he says. “How did nature make an electrical signal go from my brain to my toes so fast? The more you learn about what the ion channels have to do to make that signal, the more incredible it seems.”