Crushing Cancer’s Defenses
Vaccine approval offers hope while other armies muster
Twirling globs of white blood cells circle a tumor like a Greek army ready to lay siege. These cells are used to winning — they take down baddies such as viruses and bacteria on a daily basis. But cancer cells are not an ordinary enemy. Like Troy, they set up hefty barricades against attack, often killing white blood cells or turning them off on the spot. Too often, the immune system loses this Trojan War.

But recent advances in therapeutic cancer vaccine research may provide the immune system with an offensive edge. Such therapies aren’t the same as the shots kids get before starting school to prevent measles or polio. Nor are they like antiviral vaccines, such as Gardasil, that stave off infections which can lead to cancer. Therapeutic cancer vaccines wouldn’t prevent cancer, they would treat it: training the immune system to turn its forces on a tumor already in the body with the skill of Achilles and the strength of Ajax.
At least that has been the hope. For about a decade, large clinical trials of cancer vaccine candidates have turned out mostly disappointing. But now a handful of new therapies are showing signs that the cancer vaccine effort could be on the brink of big breakthroughs.
“The whole field is a lot more encouraged now,” says Jay Berzofsky, chief of the National Cancer Institute’s Vaccine Branch in Bethesda, Md. “It warrants a lot more investment.”
After a long search, scientists have hit on a few possible vaccines that seem to do some good. In 2010, the U.S. Food and Drug Administration gave the final OK to its first cancer vaccine, a prostate cancer therapy called Provenge. A second treatment now in large clinical trials could revive fading hopes for a melanoma vaccine. At the same time, many scientists now acknowledge that vaccines probably can’t do the job alone. Two new therapies, which aren’t vaccines but do duck tumor defenses and ultimately spur on the immune system, show promise in their own right and may make natural allies for cancer vaccines.
Though the gains have yet to match many doctors’ hopes, Provenge and other immune-energizing drugs have given terminal cancer patients months of life as part of clinical trials. “That’s when you know the idea is no longer just an attractive idea,” says Glenn Dranoff, an oncologist at the Dana-Farber Cancer Institute in Boston. “Now, you have proof.”
Coley’s vaccine
The proof may be new, but the idea behind cancer vaccines is not. A New York City surgeon named William Coley was enthralled in the late 1800s by the story of a male cancer patient who came down with a severe infection, then saw his tumor shrink dramatically. Coley decided to put the power of the fever to the test, injecting cancer patients with shots of killed pathogens, including strep bacteria. The strange thing: In many patients, it worked.
“These were patients with advanced inoperable cancer,” says epidemiologist Stephen Hoption Cann of the University of British Columbia in Vancouver. “They would be considered, by and large, incurable by today’s standards.”
Still, Coley’s findings didn’t gain much traction; instead radiation and chemotherapy became the hot treatments in oncology. But by the 1980s and ’90s, researchers were frustrated because such therapies couldn’t slow many malignant diseases. More and more teams turned to Coley’s old battle plan.
The current thinking is that infections kick the immune system into overdrive. White blood cells go after pathogens in force, causing a lot of collateral damage to tumors in the process. Today’s proposed vaccines are better sharpshooters than Coley’s original cocktail, targeting tumors specifically or at least limiting the damage in healthy organs. Some vaccines include whole cancer cells killed with radiation, while others contain a brew of proteins native to tumors. A few package such proteins into viruses.
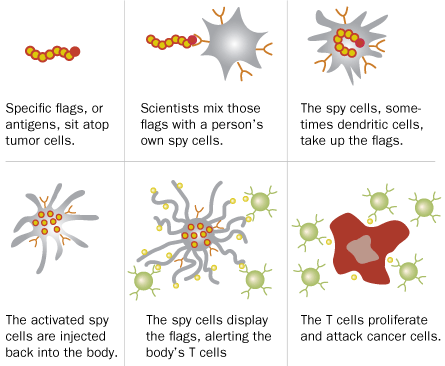
To rev up the body to attack, many of these new vaccines focus on activating immune players called dendritic cells. Among other roles, these spy cells gather intelligence on potential baddies, from free-floating proteins to whole parasites. These spies return worrisome finds to a type of white blood cell called T cells. T cells, the soldier cells, then divide rapidly and go on the offensive, looking for cells or substances that match the spies’ intel.
But because cancer cells grow from normal tissue, they often look like good guys. To clue the spy cells in to big tumors, oncologists need to fix the intel.
Provenge and beyond
And that’s exactly what the team that designed the cancer vaccine Provenge did. Doctors prepare the vaccine cocktail by taking dendritic cells and similar immune players directly from a patient and mixing them with proteins that sit atop prostate cells, says Philip Kantoff, an oncologist with Dana-Farber. The mixture goes back into the patient’s bloodstream where, scientists think, the dendritic spies present the prostate proteins to immune soldier cells. That convinces the immune system to treat the prostate cells as an enemy, like it would a common virus. Vaccine researchers had previously tried this strategy and a range of others with little success. But Provenge differed from the long list of misfires in one key respect: “It actually worked,” Kantoff says.
In a Phase III trial — a large, controlled study, often the last step before FDA approval — Kantoff and his colleagues dosed 330 prostate cancer patients with customized versions of Provenge. The team also treated 167 patients with a placebo. Patients received traditional therapies before and after vaccine treatment.
Individuals given Provenge survived for a median of about four months longer than placebo patients, nearly 26 months from when they enrolled in the study instead of about 22 months. The vaccine is obviously doing something right, Kantoff says. Curiously, though, tumors appeared to grow or spread equally fast regardless of the treatment, an observation Kantoff attributes to a monitoring failure.
The team published its results in July 2010 in the New England Journal of Medicine, and Provenge got the nod from the FDA later that year.
Before Provenge, the prospects for cancer vaccines looked grim, says Guido Forni, an immunologist at the University of Turin in Italy. “The field was very depressed.” But today, he says, it’s a different story. “Many, many people who were skeptical of vaccines are now inspired by this research to work again.”
But despite the advances, the benefits have been modest, Forni acknowledges. Provenge is better than chemotherapy alone, which gives about a two- to three-month bump to survival, but not by much. Vaccines like Provenge also rely on a patient’s own cells, and that customization can get pricey, says Leisha Emens, an oncologist at Johns Hopkins University. Next-generation treatments that tip off spy cells in the body with general ingredients from the lab could be thriftier options, she says.
One of many new therapies that takes this approach has its sights set on skin cancer. Every year, 132,000 people worldwide develop malignant melanoma, according to a World Health Organization estimate. And for many the outlook is poor. Advanced melanoma sufferers survive for a median of only six months from diagnosis. Today, “the melanoma vaccine situation is pretty barren,” says Jeffrey Weber, a melanoma specialist at the Moffitt Cancer Center in Tampa, Fla.
Weber, however, has some hope for the MAGE-A3 vaccine, a drug developed by the pharmaceutical company GlaxoSmithKline that he has worked with in the clinic. The treatment is a one-size-fits-all cocktail of flags mimicking proteins found on tumor cells (but not normal tissue) mixed with a few immune-boosting chemicals. Though the company has focused largely on melanoma and lung cancer, the same protein flags sit on a laundry list of other cancer cells, says Vincent Brichard, head of GlaxoSmithKline’s Belgium-based immunotherapeutics team. That means, if it works in further studies, the vaccine could one day treat a range of diseases including liver and bladder cancer.
Unlike Provenge, GSK’s shot hasn’t proved itself on the big stage. In a preliminary trial including 182 patients who underwent lung tumor removal surgery, tumor recurrence was delayed by 33 percent in MAGE-A3 recipients compared with those given placebo. But the improvement could have been due to chance. Brichard will be better able to evaluate the drug’s potential for success during two ongoing Phase III trials — one for melanoma and one for lung cancer — that look at thousands of patients.
Early trials have already unearthed an encouraging detail, says Brichard. A suite of genes in tumors may reveal which patients will respond well to the vaccine and which won’t. These cues could help oncologists tailor therapies on a patient-by-patient basis, often considered the “Holy Grail” of medicine, he says. Assuming the genetic signatures stack up in future studies, that sort of predictive power would make any vaccine researcher salivate.
So far, few melanoma vaccines have lived up to expectations, says Yvonne Saenger, a melanoma specialist at the Mount Sinai School of Medicine in New York City. MAGE-A3 may or may not be different, she says. She is waiting to see the Phase III results before weighing in.
Even vaccines that end up getting the FDA’s approval can do only so much, Weber says. “Getting a vaccine alone to work in metastatic melanoma, I don’t think it’s going to happen,” he says.
One big problem may be that tumors are just too good at rebuffing attacking white blood cells. And as the legendary Greeks learned, if you can’t overcome Troy’s defenses, you might as well go home.
Breaking barricades
During their day-to-day surveillance, white blood cells cull the low-hanging fruit well — cancer cells that readily give up their identities or that lack good defenses. But some cells avoid attack, and they can grow into tumors. The National Cancer Institute’s Berzofsky compares the immune system’s task to that of the Department of Homeland Security: “You can stop 99 percent of the terrorists. But it’s the 1 percent that get through that kill you.”
Often cancer cells escape the siege by taking advantage of the body’s hesitancy to attack its own cells. Despite the best laid plans in a healthy body, some white blood cells do wind up targeting healthy lungs, skin or liver. When that happens, the immune system sets off fail-safes that silence or kill outright the rogue white blood cells. Many cancers call off attacks by triggering the same fail-safes. And that’s just the start: Some tumors are downright devious, surrounding themselves with minefields laced with anti-immune chemicals. “Tumors feather their own beds,” Berzofsky says.
Cancer vaccines need a Trojan Horse to overcome these barriers. Enter ipilimumab. This drug snags onto and turns off a type of immune protein called CTLA-4. In healthy people, CTLA-4 is a godsend because it’s a natural brake on immune attacks, says Steven O’Day, director of the Melanoma Program at the Angeles Clinic and Research Institute in Santa Monica, Calif. This protein pops up on top of soldier immune cells when the cells go into battle mode. If a passing spy cell grabs onto the protein, it acts like a kill switch on the soldier cell. That’s perfect for viral infections, O’Day says. Soldier cells can hit cold and flu viruses fast and hard, then, as part of the immune system’s checks against collateral damage, dial back when the job is done.
But such short skirmishes don’t often work on tumors. “Here you’re trying to build an army of T cells, basically, to go into battle,” O’Day says. “It can take anywhere from three to six months.” Ipilimumab’s trick is to keep immune cells going.
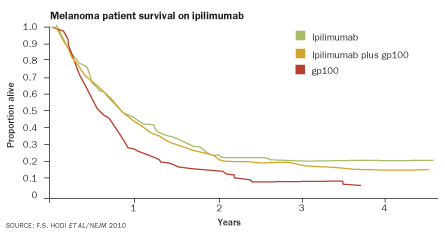
In a recent Phase III trial in patients with advanced melanoma, ipilimumab seemed to give soldier cells that much-needed breathing room, says O’Day, one of the study coauthors. Among nearly 700 patients, those that got ipilimumab survived for a median of 10 months after enrollment in the study, while patients not receiving the treatment survived for just over six, the team reported last year in the New England Journal of Medicine. But even more exciting, O’Day says, about 20 to 25 percent of patients survived for much longer — two years and counting. Ipilimumab seems to be a rarity, a drug that actually slows down melanoma.
But the drug has its downsides. In the absence of CTLA-4, many immune cells don’t know when to quit. In the Phase III trial, 10 to 15 percent of patients on ipilimumab developed severe immune side effects from diarrhea to skin rashes, and seven patients died as a result. Preparation and careful monitoring in the future should keep such ill effects in check, Saenger says.
Ipilimumab received the thumbs-up vote from the FDA in March 2011. And that’s a big deal, says Suzanne Topalian, a melanoma specialist at Johns Hopkins. “But at the same time, we all realize that ipi is a starting point,” she says. Future work will focus not only on better drugs but also on drugs that work as part of a multifront war.
Data from mouse studies suggest ipilimumab, now marketed as Yervoy, could help next-gen vaccines slip past cancer walls, says Topalian, who has helped conduct clinical trials on ipilimumab. “A vaccine is going to focus the immune response,” she says. “But these other maneuvers are going to enhance the immune response above a threshold that’s needed for clinical activity.”
Early data suggest medications that target a kill switch called PD-1, similar to CTLA-4, could pair well with vaccines and have fewer side effects, Topalian says.
So far, though, successfully mixing and matching vaccines with such kill-switch blockers remains little more than an attractive idea. O’Day’s team tested ipilimumab alongside a cancer vaccine — the gp100 vaccine — that hadn’t received FDA approval. Ipilimumab, however, seemed to do better on its own. Some doctors, including Topalian, suggest that gp100 may not be ideal, but think that newer pairings will succeed where this run failed.
And traditional drugs and treatments such as chemotherapy may work along with vaccines to make for a one-thousand-ship army. “Everyone is now on this bandwagon of developing combination therapy,” Topalian says.
Still, it may take years for patients to see the full fruits of this research, just as it has already taken years to see a cancer vaccine that holds promise. “It’s still hard because it’s still just a long path,” Saenger says. “You see a patient that has melanoma now; it’s still very difficult.” But she says there is a lot of room for hope.
A motley army now appears to be mustering. As more and more possible treatments trickle into the market, deciding which troops to send in to which patients, says O’Day, will make the fight against cancer more of an art.